
View our comprehensive portfolio of products for drug development and patient diagnosis and monitoring
Our products are trusted worldwide in research, clinical, and commercial laboratory settings.
Functional and biomarker assays for the exploration of every angle of the complement system
Our cell-based bioassays are based on iLite® technology – a cleverly designed cell-based assay system with reporter gene readouts
AAV Neutralizing Antibody (NAb), Total Antibody (TAb), and Complement Activation assays designed to provide a comprehensive view of vector immunogenicity
Solutions for detecting and semi-quantitating IgG antibodies against CCP
Our extensive CRO Services offer bioanalysis, biomarker discovery, and GMP QC testing.
With over 30 years of experience in autoimmune diagnostics, our ISO15189-certified specialist laboratory can help you reach the correct diagnosis. We also provide testing services and support for therapeutic drug monitoring.
Let us develop a custom iLite® cell line tailored to your needs.
Our cell services are highly reliable and innovative, tailored to your manufacturing needs


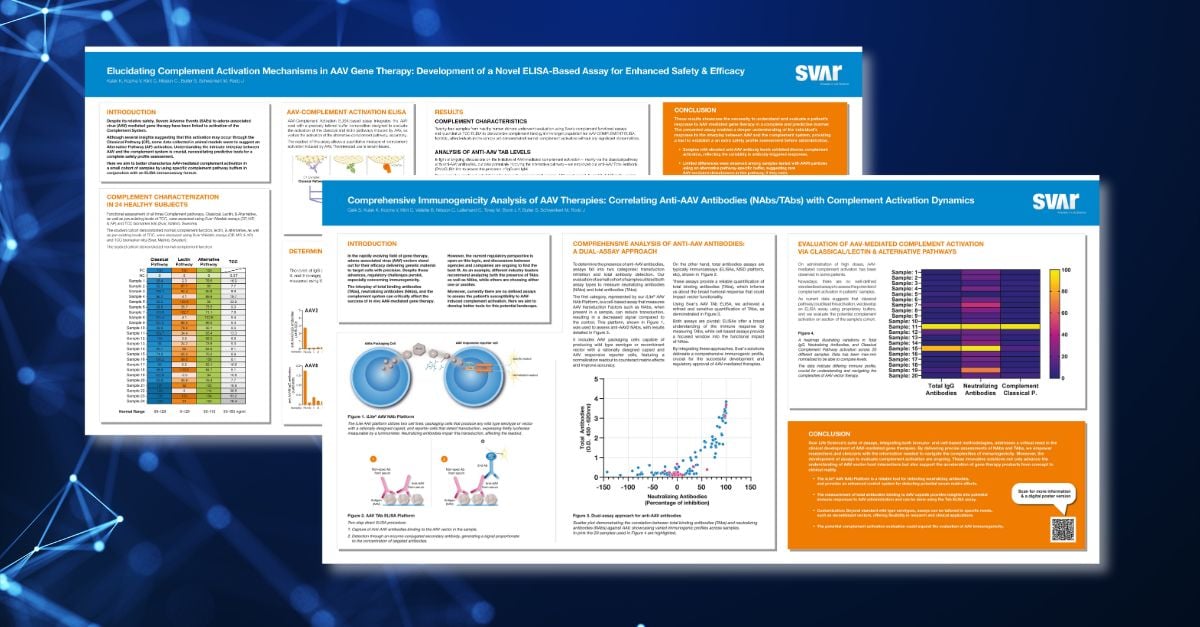
Discover how integrating immuno- and cell-based methods meets the critical need in AAV-mediated gene therapy development by accurately assessing NAbs and TAbs in our new scientific posters. You will also gain insights into predictive immunogenicity assessment and the importance of comprehensive patient response evaluation.
Download our scientific posters today and unlock a wealth of insights into AAV-mediated gene therapy development. Simply fill out the form below to access the download links.
Celik S, Kulak K, Kozma V, Klint C, Vallette B, Nilsson C, Lallemand C, Tovey M, Bovin L F, Butler S, Schwenkert M, Rodo J
Learn more about how combining immuno- and cell-based methodologies, effectively addresses the crucial demand in the clinical development of AAV-mediated gene therapies by providing precise assessments of NAbs and TAbs.
Kulak K, Kozma V, Klint C, Nilsson C , Butler S, Schwenkert M, Rodo J
Gain deeper insights into the predictive assessment of AAV-mediated immunogenicity and how understanding and evaluating patient responses to AAV-mediated gene therapy comprehensively is crucial.