
View our comprehensive portfolio of products for drug development and patient diagnosis and monitoring
Our products are trusted worldwide in research, clinical, and commercial laboratory settings.
Functional and biomarker assays for the exploration of every angle of the complement system
Our cell-based bioassays are based on iLite® technology – a cleverly designed cell-based assay system with reporter gene readouts
AAV Neutralizing Antibody (NAb), Total Antibody (TAb), and Complement Activation assays designed to provide a comprehensive view of vector immunogenicity
Solutions for detecting and semi-quantitating IgG antibodies against CCP
Our extensive CRO Services offer bioanalysis, biomarker discovery, and GMP QC testing.
With over 30 years of experience in autoimmune diagnostics, our ISO15189-certified specialist laboratory can help you reach the correct diagnosis. We also provide testing services and support for therapeutic drug monitoring.
Let us develop a custom iLite® cell line tailored to your needs.
Our cell services are highly reliable and innovative, tailored to your manufacturing needs


Effective bioassays are crucial in pharmaceutical development for assessing potency and detecting neutralizing antibodies in immunogenicity studies.
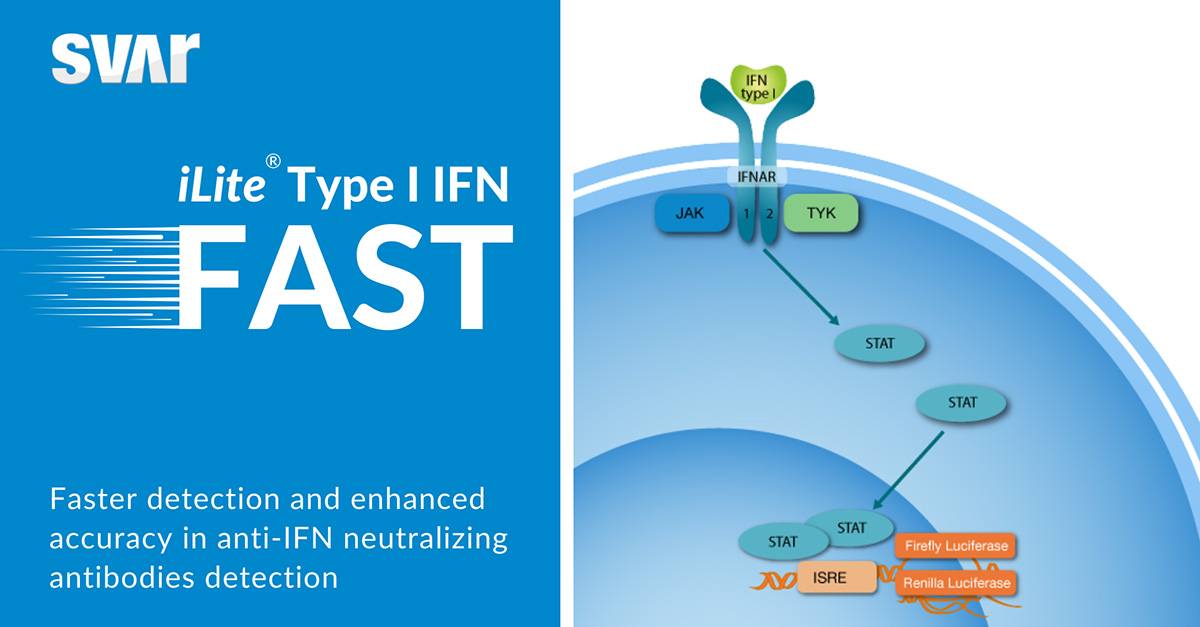
Our iLite IFN FAST second-generation cells offer shorter incubation times, Renilla normalization, and an assay-ready format for a rapid and convenient workflow. These advancements streamline your processes and improve data reliability, enabling you to make informed decisions faster and more effectively.
iLite Type I IFN FAST detects neutralizing antibodies towards interferon beta with a shorter assay time (5 h incubation) than first-generation iLite Type I IFN (18 h incubation).
This acceleration not only speeds up the overall development timeline but also allows for more timely adjustments and improvements to therapeutic strategies.
iLite Type I IFN FAST offers the opportunity to normalize type I IFN-induced firefly luciferase activity using constitutively expressed renilla luciferase.
This normalization process enhances the accuracy and consistency of NAbs detection, ensuring that results are more dependable and reproducible.
By integrating faster NAb antibody detection with Renilla normalization, the second-generation iLite cells offer a powerful tool for pharmaceutical development.
iLite Type I IFN FAST cells come in an assay-ready format, eliminating the need for time-consuming cell culture procedures, and saving both time and money.
You can start the experiments immediately without the hassle of cell thawing, counting, or passaging.