
Introducing the new iLite® C3a Assay Ready Cells
A new way to study the Complement System
Building on Svar’s expertise in cell-based complement assays, we introduce the iLite® C3a Assay Ready Cells, a new Mode-of-Action (MoA)-reflective assay for functional studies of C3a and its receptor C3aR. The iLite C3a Assay Ready Cells are optimized for potency testing in drug development, through receptor-specific readout of C3a activity, and for complement pathway research. This Research Use Only (RUO) cell line joins Svar’s unique portfolio of cell-based complement assays including the iLite® C5a Assay Ready Cells and the iLite® CD20 (+) Svar Luc Assay Ready Cells for complement-dependent cytotoxicity (CDC) studies.
As the focus on developing complement therapeutics grows, with the aim of delivering meaningful benefits to patients, researchers face significant challenges due to the inherent complexity of the complement system and the risk of unintended side effects. Highly specific, MoA-reflective cell-based assays have proven to be indispensable in successfully identifying and developing the most promising drug candidates that can deliver the desired therapeutic effect.
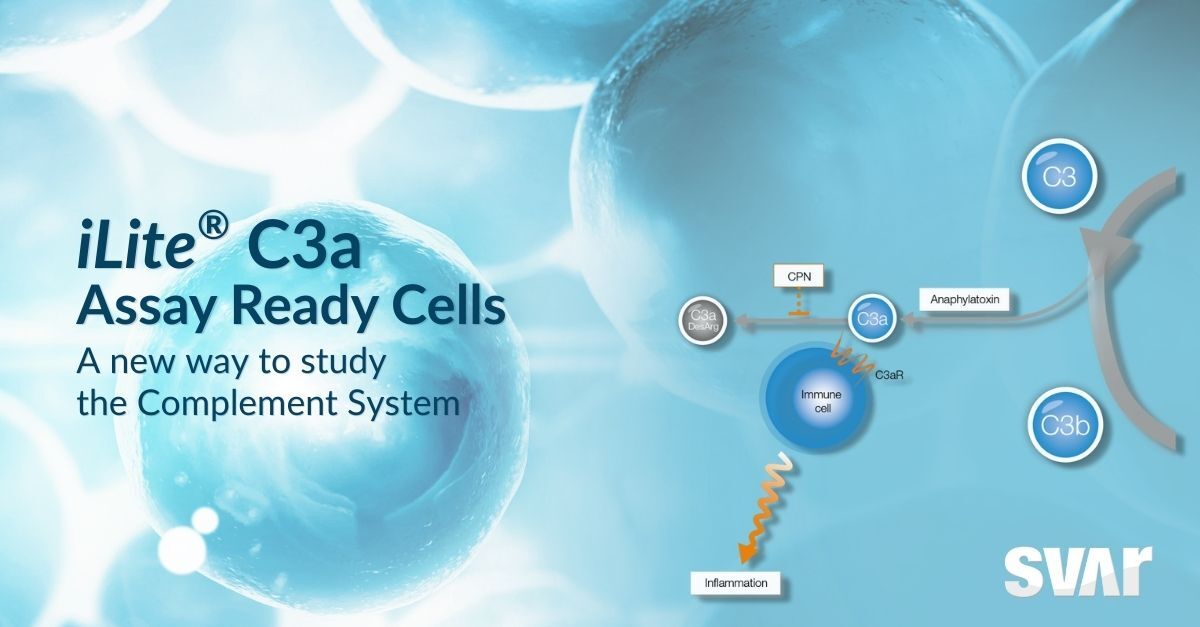
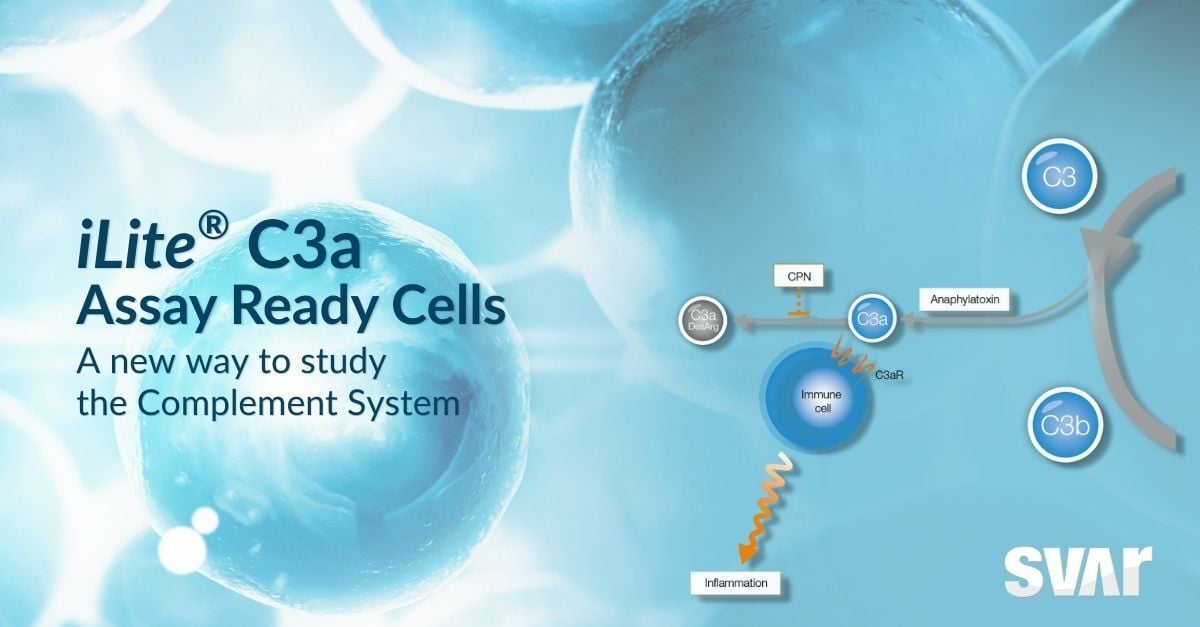
The new iLite C3a Assay Ready Cells enable a precise assessment of the C3a peptide levels through a receptor-specific luminescent readout. Thanks to its high specificity for C3a, the assay is free from the cross-reaction to C3a des-Arg and other components of the cascade that affects other assays.
With robust consistency over time and batches, this cell line is an ideal solution for potency studies of therapeutics that aim at modulating C3a-mediated inflammatory and immune responses. This includes C3aR antagonists and drug candidates acting upstream of C3 cleavage in the complement cascade. C3a is also an essential biomarker and indicator molecule for the evaluation of efficacy and potency of drug candidates acting upstream in the complement cascade through different therapeutic strategies.
With the addition of iLite C3a Assay Ready Cells, our cell-based solution for complement studies enables unique MoA-reflective mechanistic studies of two important biomarkers in the complement cascade, C5a C3a, as well as cytotoxicity studies (CDC).
iLite® C3a Assay Ready Cells
- High specificity for C3a: receptor-specific readout of C3a activity via luminescence with no cross reactivity for C4a, C5a and C3a des-Arg, allowing for reliable discrimination between complement components.
- Reliable consistency: the excellent sensitivity and low variation ensure reliable and comparable functional readouts when performing continuous assays over time and multiple lots.
- Optimized for potency studies: the cell line is well-suited for delivering MoA-reflective reproducible, high-throughput testing of complement activation in pure protein contexts. It is a perfect tool for the development of drug candidates that interact with the C3a receptor, C3aR, or that modulate the levels of C3a by acting upstream of C3 cleavage in the complement cascade.
- Tailored for generating robust evidence: fit for purpose for drug development and for generating evidence to be included in market approval submissions to regulatory bodies.
- Simplified workflow and fast results: the C3a reporter-gene assay-ready format removes the need for cell culturing, delivering results in approximately five hours, enabling a fast, streamlined work process with low variability.
Combine it with:
WIESLAB® Complement Screen (RUO)
iLite® C5a Assay Ready Cells
iLite® CD20 (+) Svar Luc Assay Ready Cells
Learn more about C3a and the Complement System
The complement system is a key part of the innate immune system and plays an important role in the immune response, defending the body against infections and regulating inflammation response. With over 40 proteins involved in three different pathways, the complement system is a promising target for therapeutics in inflammatory and autoimmune diseases. Deficiencies or overactivation of the different components of the complement cascade can result in damage to cells and tissues, ultimately causing harm to the body.
The C3a and C5a peptides are anaphylatoxins involved in the inflammatory and immune response and in other key activities including chemotaxis in immune cell activation. They are produced through proteolytic cleavage of the C3 and C5 proteins, two central components in the complement cascade.
C3a works as a signaling effector, contributing to modulate the inflammatory and immune response via interaction with the specific receptor C3aR. While levels of C3a are always detectable in plasma, marked increases in its concentration are observed during inflammation. The activity of C3a is physiologically regulated by plasma carboxypeptidases, which transforms it into C3a des-Arg, a less-potent form with reduced affinity to C3aR.
Modulating C3a availability or its interaction with C3aR can play an important role in the development of therapeutics that modulate the inflammatory and immune responses. Regulating C3 cleavage upstream in the complement cascade directly affects the levels of C3a release, while C3aR antagonists can interfere with the interaction between C3a and C3aR.