iLite® Solutions for AAV Antibody Assessment
In the dynamic landscape of gene therapy, understanding immunogenicity is paramount, and here, our iLite AAV NAb Assay platform emerges as your strategic ally. Developing AAV capsids for gene therapy that go unnoticed by the immune system is challenging; getting the tools to support your vector design is key.
Discover our iLite® AAV NAb platform – the key to uncover the silence of AAV serotype capsids!
For a deeper insight into gene therapy and its potential benefits and challenges, click here.
A two-cell line setup orchestrated with precision
The different iLite packaging cells produce AAV2, AAV5, AAV6, AAV8, and AAV9 vector serotypes, and the AAV Responsive Reporter cells, trigger the expression of Firefly luciferase in response to AAV transduction. This AAV transduction correlates directly with the produced readout, easily measurable by a standard luminometer.
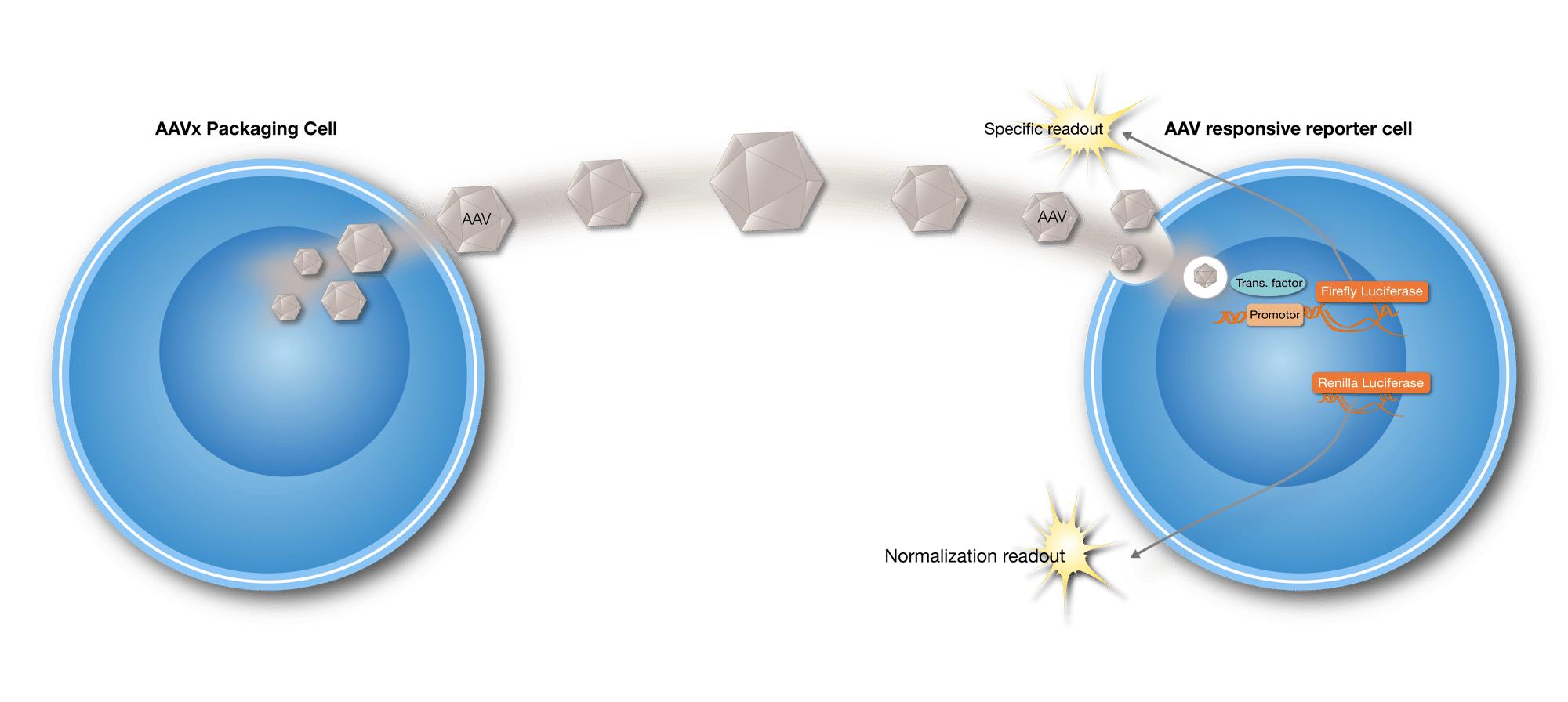
Principle of the AAV Platform. Our two-cell line setup with AAV-producing Packaging cell and AAV-responsive Reporter cells produce and detect AAV vector transduction, respectively.
In the presence of neutralizing antibodies, the AAV transduction is impaired, resulting in a lower production of the specific readout.
In addition, the AAV Responsive Reporter cells offer an added layer of precision. With a constitutively expressed second reporter gene, Renilla Luciferase. These cells can serve as an internal control of matrix effects. This not only allows for the determination of biological sample cytotoxic effects but also ensures results independent of the number of cells used in the assay. This dual luciferase expression capability enhances the robustness and reliability of our platform.
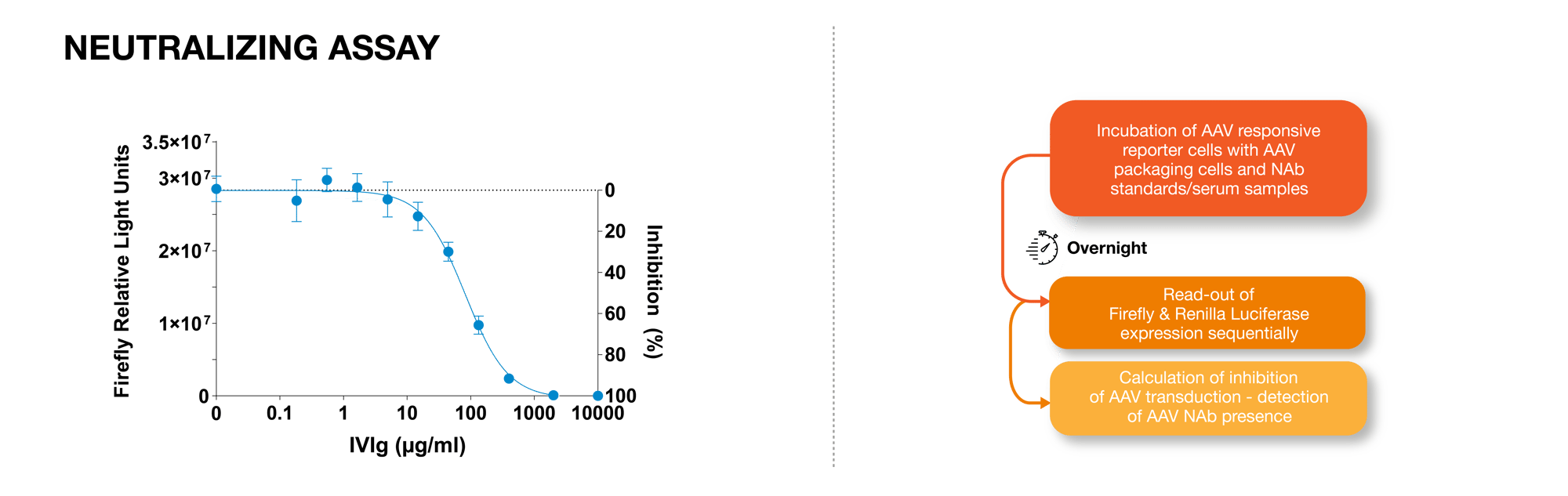
How does the AAV Platform work? Incubation of AAV responsive reporter cells with the recommended number of AAV packaging cells and NAb standards or serum sample. Incubate overnight. Read out Firefly and Renilla Luciferase expression sequentially. Calculation of inhibition of AAV transduction - detection of AAV NAb presence.
Why Immunogenicity Assessment Matters
Gene therapy is a revolutionary medical approach that modifies host cell DNA to treat and prevent diseases. At its core, gene therapy involves the precise alteration, addition, or deletion of genetic material to correct or modify the function of genes. It is predominantly carried out using different vector types to insert the genetic material.
To ensure the effectiveness and safety of gene therapy, advanced bioassays are required to determine the immunogenicity of the vector and genetic cargo.
One common carrier is AAV vectors; AAVs are non-pathogenic, have low immunogenicity, and efficiently deliver genetic material to both dividing and non-dividing cells, making them attractive for therapeutic applications.
It is essential to develop AAV capsids that can evade detection by the immune system, as pre-existing anti-AAV neutralizing antibodies (NAbs) can obstruct transgene transfection, thus making gene therapy ineffective.
Since many individuals have been exposed to the wild-type AAV virus, with up to 90% having prior exposure, often in early childhood, approximately 50% of the population typically carries antibodies against the different AAV serotypes. This underscores the critical need for accurate immunogenicity assessment.
Key Features of the iLite AAV NAb platform
Customization for Any Capsid
The flexibility and adaptability of iLite technology in this platform means that our AAV-packaging cells can be easily adapted to produce any vector of choice and be a critical tool in the assessment of recombinant next-gen vectors, offering flexibility for hybrid, chimeric, or semi-synthetic capsids.
Click here to contact us about the development of your unique vector
Efficient Transduction Inhibition Assay
The AAV responsive cell line, in combination with our novel-engineered AAV vector-producing packaging cell, eliminates the need for vector production, resulting in the reduction of lot-to-lot variation, ensuring a streamlined workflow, accuracy, and reliability.
Consistency in Every Vial for Robust And Reproducible Results
The assay-ready format of the AAV packaging and responsive cell guarantees consistent cell numbers collected and frozen at the same passage in each vial. This cryopreservation process enables high-quality results, ideal for robust and reproducible results. This empowers informed decision-making and optimization of AAV capsids.

Reduced Incubation Time for Fast and High-Quality Results
The AAV packaging and responsive cells are available in a convenient frozen, thaw, and ready-to-use format, eliminating the need to culture them. This results in a short turnaround time of just overnight from plating the cells to results, allowing for efficient and timely assessment of NAbs.
"The iLite technology, with its proven track record in measuring NAbs against biological drugs, is a great tool to evaluate the ability of antibodies to block AAV transgene expression."
Dr. Ulrich Mayer, Global Technology Manager, Cell-based solutions
Utilize Our Expertise as Your CRO services partner
Our expertise in immunogenicity and neutralizing antibodies makes us an ideal partner for outsourcing these assays. We offer comprehensive support for custom immunogenicity and transduction inhibition assays tailored to meet your AAV-based therapy needs. Our team works collaboratively with yours to ensure precision and efficiency, advancing your gene therapy initiatives.