
Our Products Provide Answers in Life Science

Our Products Provide Answers in Life Science
All Products
We provide innovative solutions that deliver reliable, accurate, and easy-to-use tools for drug development, basic research, clinical applications, and diagnostics. Our portfolio combines advanced technologies with proven methods to support critical decision-making across research, development, and patient care.
Products to support your research
Based on the powerful iLite® technology, our cell-based solutions offer excellent sensitivity and specificity, combined with robustness and flexibility, enabling truly fit-for-purpose cell-based assays.
Our complement assays consist of highly specific, ready-to-use ELISA and cell-based assays for functional and quantitative testing. Built for both manual and automated workflows, they deliver objective and reproducible results in just a few hours – minimizing variability while ensuring consistent data you can trust.
We offer CCP solutions that provide a powerful tool for the early and accurate detection of rheumatoid arthritis.
Together, our products reflect our commitment to quality and innovation, giving you confidence in every result.
Our Product Portfolio
FILTER VIEWComplement C4d
Detect complement activation via the classical and lectin pathways with this C4d biomarker ELISA
Complement Factor P Functional Assay
Zoom in on the amplification loop of the alternative complement pathway with this Factor P functional ELISA
Complement Factor P Quantitative Assay
Zoom in on the amplification loop of the alternative complement pathway with this Factor P quantitative assay
Complement TCC
Measure terminal complement complex formation to evaluate full pathway activation and membrane attack complex formation
iLite® IL-23 Assay Ready Cells
Precise measurement of IL-23-induced signaling in a cell-based context
iLite® TNF-alpha Assay Ready Cells
Reproducible, cell-based quantification of TNF-α bioactivity. Ideal for functional testing of anti-TNF therapeutics and mechanism-of-action studies
iLite® AAV Responsive Reporter Assay Ready Cells
Reporter cell line designed to quantify AAV vector transduction efficiency and detect NAbs that impair gene delivery
iLite® AAV2 Packaging Assay Ready Cells
Engineered and optimized to produce AAV vectors of serotype 2 for use in NAb detection studies
iLite® AAV5 Packaging Assay Ready Cells
Engineered and optimized to produce AAV vectors of serotype 5 for use in NAb detection studies
iLite® AAV6 Packaging Assay Ready Cells
Engineered and optimized to produce AAV vectors of serotype 6 for use in NAb detection studies
iLite® AAV8 Packaging Assay Ready Cells
Engineered and optimized to produce AAV vectors of serotype 8 for use in NAb detection studies
iLite® AAV9 Packaging Assay Ready Cells
Engineered and optimized to produce AAV vectors of serotype 9 for use in NAb detection studies
iLite® ADCC Effector FcγRIIIa (V)
Designed to quantify ADCC activity
iLite® ADCP Effector FcγRIIa
Accurate measurement of ADCP activity
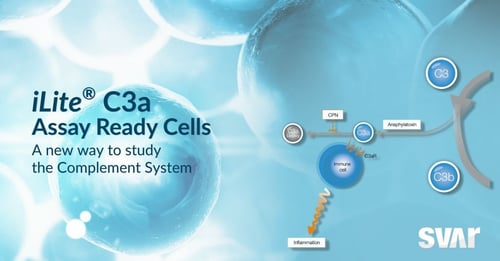
iLite® C3a Assay Ready Cells
Quantitative measurement of complement C3a activity in a cell-based format
iLite® C5a Assay Ready Cells
Quantitative measurement of complement C5a activity in a cell-based format
iLite® CD19 (+) Target
Engineered to express CD19, this cell line is ideal for ADCC and bispecific antibody assays
iLite® CD19 (-) Target
CD19-negative target cell line used as a specificity control in ADCC and T cell engager assays
iLite® CD20 (+) Svar Luc Assay Ready Cells
Designed for sensitive and reproducible assessment of CDC activity
iLite® CD20 (+) Target
Target cells expressing CD20 for use in ADCC and ADCP bioassays
iLite® CD20 (-) Target
CD20-negative target cells. Ideal for use as controls in CD20-specific bioactivity assays
iLite® CD3 Effector
Optimized for quantifying T cell-mediated cytotoxicity and bispecific antibody function via CD3 engagement
iLite® EGFR (+) Target
Well-suited for ADCC or bispecific antibody assays targeting EGFR-positive cells
iLite® EGFR (-) Target
A negative control cell line for EGFR-targeted bioassays
iLite® FGF-21 Assay Ready Cells
Cell-based measurement of FGF-21 signaling, supporting metabolic research and development of FGF-21 analogs or mimetics
iLite® G-CSF Assay Ready Cells
Reliable cell-based quantification of G-CSF activity, supporting biosimilar development and batch consistency testing
iLite® GM-CSF Assay Ready Cells
Functional quantification of granulocyte-macrophage colony-stimulating factor activity
iLite® hCG Assay Ready Cells
A sensitive, cell-based method for quantifying hCG bioactivity
iLite® HER2 (+) Target
Target cells for functional antibody testing in HER2-targeted bioassays
iLite® HER2 (-) Target
HER2-negative target cell line used as a control in HER2 bioactivity, ADCP, and ADCC assays
iLite® IL-12 Assay Ready Cells
Functional, cell-based quantification of IL-12 signaling
iLite® IL-2 Assay Ready Cells
Sensitive quantification of IL-2 activity or inhibition
iLite® IL-6 Assay Ready Cells
Reproducible, cell-based detection of IL-6 bioactivity
iLite® Insulin Assay Ready Cells
Robust, quantitative measurement of insulin receptor activation, facilitating characterization of insulin analogs and biosimilars
iLite® mTNF-alpha (+) Target
Target cells for functional characterization of TNF-targeting biologics
iLite® mTNF-alpha (-) Target
A control cell line used for specificity assessment in TNF-targeted assays
iLite® mVEGF (+) Target
Target cells for potency assays of anti-VEGF agents
iLite® mVEGF (-) Target
Negative control cell line for VEGF-targeted assays
iLite® RANKL Assay Ready Cells
Precise quantification of RANKL activity or blockade in a cell-based system
iLite® TLR4 Assay Ready Cells
Detection of TLR4-mediated NF-κB signaling
iLite® TLR9 Assay Ready Cells
Cell-based measurement of TLR9 pathway activation
iLite® TNF-alpha Xcel Assay Ready Cells
Pre-qualified and assay-ready, these cells provide rapid and sensitive detection of TNF-α activity
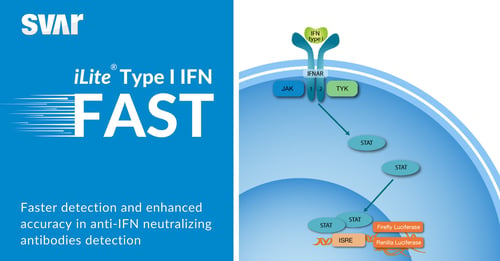
iLite® Type I IFN FAST Assay Ready Cells
Accelerated quantification of type I interferon signaling with a shortened incubation time
iLite® VEGF Assay Ready Cells
Quantification of vascular endothelial growth factor activity with high sensitivity
IMMUNOSCAN CCPlus®
ELISA for determining IgG antibodies to CCP in human sera
IMMUNOSCAN CCPlus® - RUO
"Research use only" version ELISA for determining IgG antibodies to CCP in human sera
WIESLAB® Complement Alternative Pathway
Analyze specific activation of the alternative pathway with this functional ELISA
WIESLAB® Complement Alternative Pathway (RUO)
Research-use-only ELISA for profiling alternative complement pathway activation
WIESLAB® Complement Classical Pathway
Quantify the activation of the classical complement pathway
WIESLAB® Complement Classical Pathway (RUO)
Sensitive analysis of classical pathway activation
WIESLAB® Complement MBL Pathway
Explore the activation of the lectin complement pathway
WIESLAB® Complement MBL Pathway (RUO)
RUO ELISA for pathway-specific detection of MBL-mediated complement activation
WIESLAB® Complement Screen
Gain a complete picture of complement activation across all three pathways
WIESLAB® Complement Screen (RUO)
A RUO-format screening assay that evaluates activation of the classical, lectin, and alternative pathways
Didn't find what you were looking for?
We offer products customized to your needs. Our custom iLite® reporter-gene assays can be adapted to almost any target. We also offer custom ELISA plates and other products. In addition, you can take advantage of our service offerings and let us do the work for you.
485
Scientific papers with our products
30+
Years of product development experience
We deliver answers in life science
News


-1.png?width=500)
.jpg?width=500)
/Communication%20Themes%202025/Theme%201%20-%20IVDR/Theme%201%20-%20IVDR%204.jpg?width=500)



Downloads




6 FEBRUARY 2026
Unraveling Complement’s Role in Blindness: The Work of Professor Simon Clark
Read post
6 FEBRUARY 2026
Translating Research into Care - Exploring Complement with Dr Sophie Chauvet
Read post
6 FEBRUARY 2026
Insights on Targeting Complement Dysregulation - Dr. Elena Goicoechea de Jorge
Read post



26 JANUARY 2026
20 Years of Innovation: The Legacy of the WIESLAB® Complement System Assays
Read post-1.jpg?width=500)

.jpg?width=500)

Contact Our Product Team
Write us a message and a product specialist will get in touch
