Svar News
Stay up to date with the latest developments, announcements, and expert perspectives. Get company updates, the latest life science trends, thought leadership articles, and event highlights – all designed to keep you up to date. Sign up to our newsletter



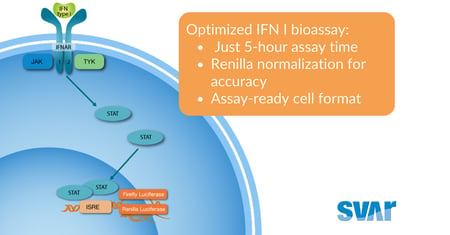
-1.png?width=450)
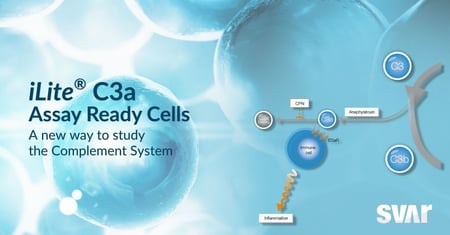
.jpg?width=450)
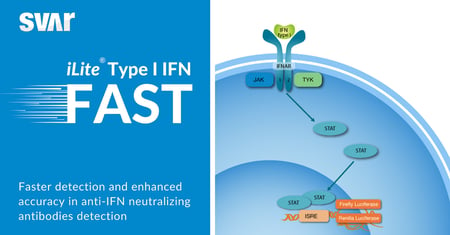
/Communication%20Themes%202025/Theme%201%20-%20IVDR/Theme%201%20-%20IVDR%204.jpg?width=450)