- Other Products
- CCP
The iLite Functional Bioassay portfolio is based on a reporter gene system and offers a convenient and powerful way of measuring and quantifying drug potency, detecting neutralizing antibodies (NAbs) and assessing immunogenicity.
Reporter gene cell lines allow for detection of signal which is directly proportional to the induced function of your target compound.
A reporter gene is inserted in the cell under an inducible promotor and the gene is expressed to give a quantifiable readout. The iLite technology utilizes the existing intra cellular system, thereby maintaining the biological relevance of the assay. A unique “lock and key” function has been developed in the iLite cell lines which amplifies the signal and makes it more specific, resulting in a more sensitive cell and a larger dynamic range.
The iLite Functional Bioassay portfolio consists of cell lines provided in an “assay-ready” format for a rapid and convenient workflow. The assay ready format also reduces assay variability, optimizing their use in screening, characterization, stability and potency studies. iLite cell lines can be tailored to virtually any biopharmaceutical target.
Biological activity is a critical quality attribute for biopharmaceuticals and a functional bioassay will, in many cases, give a more intricate answer than a traditional binding assay since the functionally of the compound can be determined, not just a binding interaction.
Because a bioassay or bioactivity assay can reflect the mechanism of action (MoA) of a potential drug, it is an important component of the complete analytical profile and functional bioassays, reporter bioassays and cell health assays are used to characterize and develop novel therapeutics all the way from high throughput screening, through CMC lot release testing, to determination of immunogenicity.
Bioassays are an important component in ensuring regulatory compliance, and effective bioassays require reproducible cell lines. Functional Bioassays based on luminescence are very popular as they have high sensitivity, offer a large dynamic range, are easy to use and quick to perform.
The added advantage of getting these cell lines in an Assay ready format gives superior Ease of use
An iLite Cell Based Assay is run – similar to an ELISA, but here the cells act as the reagent and are ready‐to-use instantly after thawing. This format gives your assay a very quick turnaround time - all the iLite assays are performed within a workday or at most overnight.
iLite® TNF-alpha [BM3044]
iLite® TNF-alpha Xcel Assay Ready Cells [BM4044]
iLite® Type I IFN FAST [BM4049]
iLite® RANKL [BM4052]
iLite® IL-2 [BM4002]
iLite® IL-6 [BM4061]
iLite® IL-12 [BM4012]
iLite® IL-23 [BM4023]
iLite® VEGF [BM4021]
iLite® FGF-21 [BM3071]
iLite® GM-CSF [BM4050]
iLite® G-CSF [BM4055]
The iLite functional reporter bioassays are used to characterize and develop novel therapeutics – from high throughput screening, through CMC lot release testing, to determination of immunogenicity.
It is a versatile tool with applications throughout the drug continuum due to its clever engineering and use in accurate functional assessments.
At an early stage in the development process, thousands of compounds need to be narrowed down to a small number of the most promising candidates.
In order to identify the best candidates, you should use an assay that as closely as possible reflects the complex processes of the cell, where different proteins or pathways are induced or inhibited depending on the circumstances. It is not sufficient to just determine whether or not the compound binds to its intended target.
Our cell-based iLite assays offer this functionality. In addition to finding out if the candidate drug binds to it target, you get additional information that can help you determine if the compound functions according to its intended mechanism of action.
Determining the potency of a candidate is one of the most essential parts of drug development.
Based on the regulatory guidelines, the potency of the drug candidate often needs to be addressed by a cell-based assay to achieve the biological relevance required. Potency assays also need to be highly reproducible.
The iLite system fits both criteria and can be used for measuring the drug candidate’s potency for Quality Control and as a batch release assay. Typically, it has to be established that the drug gives the same potency (effectiveness) for each batch when tested in a biologically relevant setting,
iLite cell lines can be successfully used to:
Immunogenicity is an immune response against a therapeutic antigen causing that medication to lose its effectiveness over time and potentially resulting in serious illness. Clearly, immunogenicity is an important factor to consider as manufacturers develop new protein therapeutics.
Because drug development becomes increasingly costly as it moves downstream, it is important to anticipate immunogenicity as far upstream as possible in the drug discovery process.
Customers assessing immunogenicity need an Anti-Drug Antibody (ADA) assay and a NAb assay. While ADAs are most easily assessed by a regular ligand binding assay, detection of NAbs most often requires a functional cell-based assay.
Requirements for NAb assays are:
This fits iLite assays perfectly. By combining regulatory compliance with excellent reproducibility and assay performance, iLite technology can circumvent many of the hurdles of traditional cell-based assays.
With many of the existing patents of monoclonal antibody block buster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization is needed to show comparability between innovator and biosimilars and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo. Here, iLite Functional Bioassay Profiles could be of great value, when assessing and comparing the response of the compounds.
The iLite functional reporter bioassays are used to characterize and develop novel therapeutics – from high throughput screening, through CMC lot release testing, to determination of immunogenicity.
It is a versatile tool with applications throughout the drug continuum due to its clever engineering and use in accurate functional assessments.
At an early stage in the development process, thousands of compounds need to be narrowed down to a small number of the most promising candidates.
In order to identify the best candidates, you should use an assay that as closely as possible reflects the complex processes of the cell, where different proteins or pathways are induced or inhibited depending on the circumstances. It is not sufficient to just determine whether or not the compound binds to its intended target.
Our cell-based iLite assays offer this functionality. In addition to finding out if the candidate drug binds to it target, you get additional information that can help you determine if the compound functions according to its intended mechanism of action.
Determining the potency of a candidate is one of the most essential parts of drug development.
Based on the regulatory guidelines, the potency of the drug candidate often needs to be addressed by a cell-based assay to achieve the biological relevance required. Potency assays also need to be highly reproducible.
The iLite system fits both criteria and can be used for measuring the drug candidate’s potency for Quality Control and as a batch release assay. Typically, it has to be established that the drug gives the same potency (effectiveness) for each batch when tested in a biologically relevant setting,
iLite cell lines can be successfully used to:
Immunogenicity is an immune response against a therapeutic antigen causing that medication to lose its effectiveness over time and potentially resulting in serious illness. Clearly, immunogenicity is an important factor to consider as manufacturers develop new protein therapeutics.
Because drug development becomes increasingly costly as it moves downstream, it is important to anticipate immunogenicity as far upstream as possible in the drug discovery process.
Customers assessing immunogenicity need an Anti-Drug Antibody (ADA) assay and a NAb assay. While ADAs are most easily assessed by a regular ligand binding assay, detection of NAbs most often requires a functional cell-based assay.
Requirements for NAb assays are:
This fits iLite assays perfectly. By combining regulatory compliance with excellent reproducibility and assay performance, iLite technology can circumvent many of the hurdles of traditional cell-based assays.
With many of the existing patents of monoclonal antibody block buster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization is needed to show comparability between innovator and biosimilars and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo. Here, iLite Functional Bioassay Profiles could be of great value, when assessing and comparing the response of the compounds.
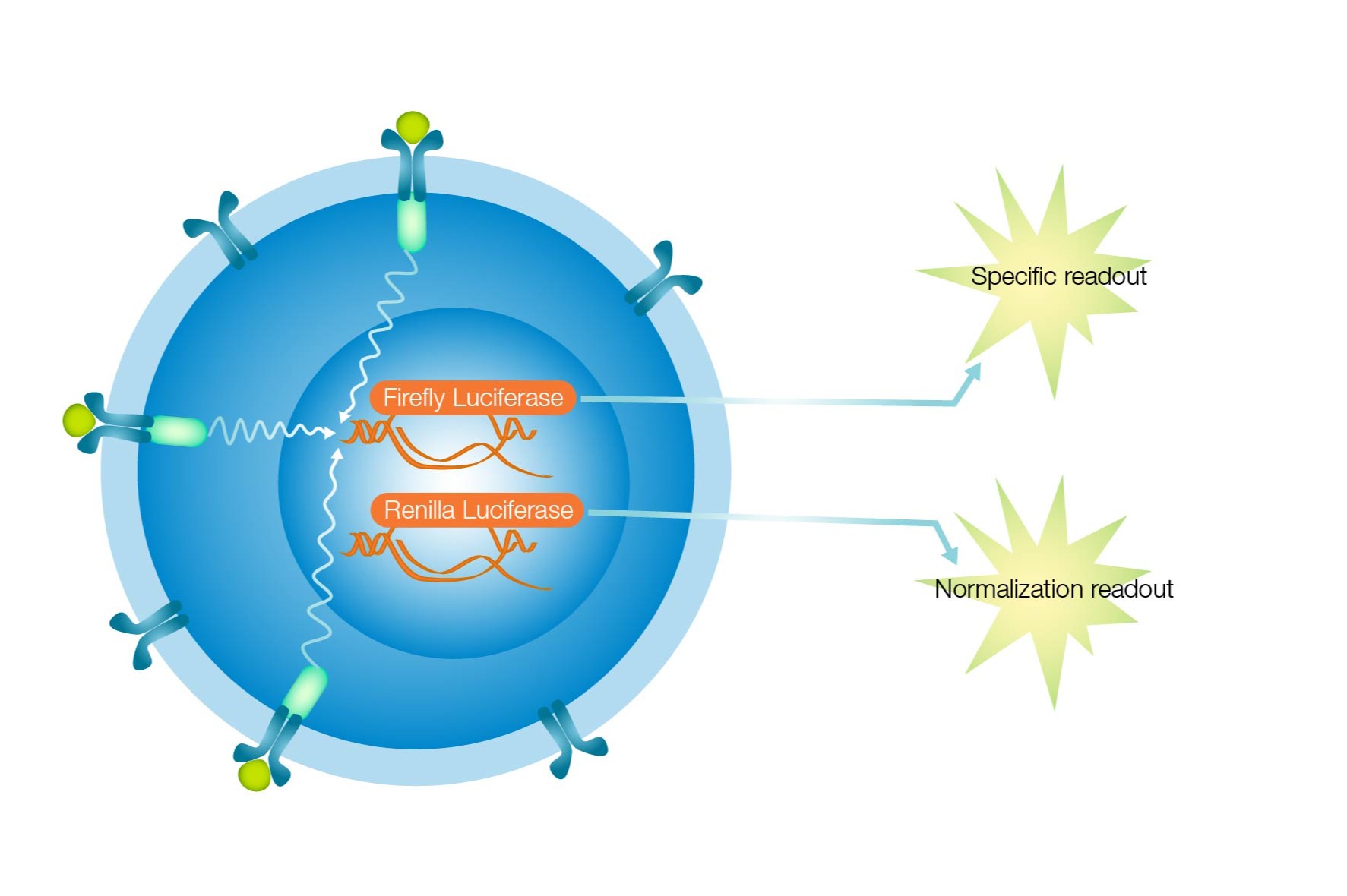
The iLite® cell-based assays are based on a simple reporter gene technology. Receptors, specific for a certain target or ligand, are expressed on the surface of a cell. Once the ligand binds to the receptor, this will trigger an intra-cellular signaling cascade, which leads down to a promoter region, fused to the reporter gene, in this case the Firefly Luciferase. Activation of the reporter gene along with the addition of a substrate will generate light. The amount of light will be correlated to the amount and activity of ligand that was bound to the receptors.
In the case of the iLite technology, there is a second reporter gene present, here shown by the Renilla Luciferase. This reporter gene is under the control of a constitutive promoter, and as such, will be active all the time in any living cell. In other words, the amount of light generated from this second reporter gene will be correlated to the number of living cells and can therefore be used for normalizing the results to compensate for differences in cell number.
The iLite technology provides a functional cell-based assay format, with many of the features and benefits normally associated with ligand binding assays. The technology is based on a reporter gene format, which has been modified and adapted for applications during the whole drug development process.
Cell-based assays are powerful tools in drug development as they can be used to determine a drug candidate’s mechanism of action in an in vitro model. The ability of cell-based assays to mimic the environment of the body have made them the preferred choice of regulatory agencies. However, cell-based assays can be challenging to set up and can have drawbacks, like high variance, which make results difficult to interpret.
Ligand binding assays are not as well suited to mimic the mechanism of action of a drug, but have other advantages, such as being drug-tolerant, sensitive and robust. In some cases, use of ligand binding assays may provide valuable information in the early steps of assessing a drug candidate. Often, the best approach is to complement a cell-based assay with a ligand binding assay.
Regardless of how you choose to proceed, Svar has the capabilities and experience to assist in your immunogenicity project. We can use our inhouse assay development to develop ligand binding assays for your target. In addition, we offer a wide selection of cell-based iLite assays. If your target is not on the list, we can create a custom assay. Both kinds of assay development are offered and run by our Bioanalytical Services.