- Other Products
- CCP

The iLite ADCC product portfolio is based on a reporter gene system and offers a convenient and powerful way of measuring the efficacy antibodies have to elicit ADCC through the CD16/ FcyRIIIa receptor in vitro.
This is achieved by combining an engineered effector cell that closely resembles the natural FcγRIIIA signal transduction pathway with homologous target cells with a controlled antigen expression (+) or depletion (-).
Taken together, our unique iLite ADCC portfolio offers unparalleled sensitivity for each target and a system that is truly greater than the sum of its parts.
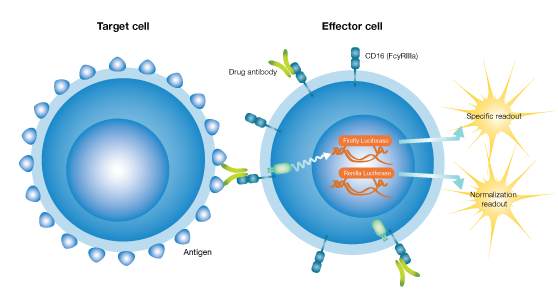
The iLite ADCC product portfolio consists of cell lines provided in an “assay-ready” format for a rapid and convenient workflow and a further reduction in assay variability, enabling their use in antibody screening, characterization, stability, and potency studies.
DOWNLOAD FREE WHITEPAPER

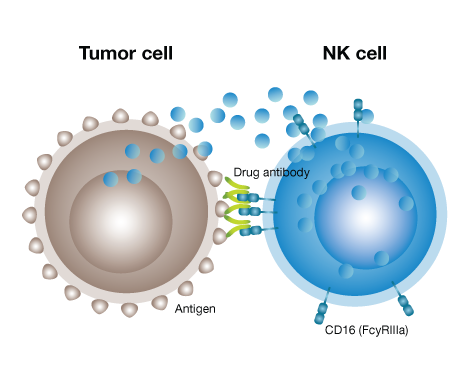
Antibody-dependent cellular cytotoxicity (ADCC) is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively destroys a target cell whose membrane-surface antigens have been bound by specific antibodies.
ADCC is part of the adaptive immune response due to its dependence on a prior antibody response. It is independent of the immune complement system that also destroy targets but does not require any other cell to do so.
The typical ADCC involves activation of an effector cell which classically is known to be natural killer (NK) cells that interact with immunoglobulin G (IgG) antibodies. Other agents such as macrophages, neutrophils, and eosinophils can also mediate ADCC.
Antibody-dependent cell-mediated cytotoxicity (ADCC) is an immune response where Fc receptor-bearing effector cells recognize and kill antibody-coated target cells that express tumor or pathogen-specific antigens on their surface. Many antibody-based therapeutics rely, at least in part, on their ability to induce ADCC in patients. The iLite ADCC product line offers a convenient and powerful way of measuring the efficacy of antibodies to elicit ADCC in vitro.
The ADCC process is triggered when the effector cell interacts with a drug antibody bound to its target cell. A Fcγllla (CD16a) receptor on the surface of the effector cell binds to the Fc region of the antibody, thus creating a bridge between the effector and target cells.
Following the formation of this bridge, the engineered effector cells will, instead of lysing the target cells as in the in vivo situation, produce luciferase through an intracellular pathway and generate luminescence exclusively from this cross-linking and signaling. The strength of the luminescence correlates to the ability of the drug to induce ADCC.
The interaction between the reporter gene effector cell and the target cell, generated by the crosslinking of the two cells via a specific drug antibody.
The resulting luminescence originates exclusively from this crosslinking and the signaling from the CD16 receptor to the Firefly Luciferase promoter.
The strength of the luminescence correlates to the drugs ability to induce ADCC.
Upon binding to the antibody, NFAT, CREB, NfκB, API, and Jak-STAT pathways are activated in the effector cell. The promoter controlling the firefly luciferase gene has been cleverly engineered to contain binding sites for all these pathways. This more closely reflects the in vivo FcγRIIIA signal transduction pathway than commercially available reporter genes that rely solely on the NFAT-responsive promoter.
Like most iLite cell lines, the iLite ADCC Effector Cells also have a secondary luciferase readout, from a luciferase gene expressed under the control of a constitutive promotor. This enables normalization of each individual readout according to cell number and compensates for potential matrix effects.
Increasing evidence suggests that ADCC plays a significant role in antibody-mediated protection and control of viral infection and several laboratory methods exist for determining the efficacy of antibodies or effector cells in eliciting ADCC.
The iLite ADCC Reporter Bioassay is designed for evaluating ADCC of therapeutic monoclonal antibodies (mAbs) and exhibits greatly reduced variability and offers an easier workflow than traditional ADCC assays.
It is an ideal assay for applications such as potency lot release and antibody screening, as well as for assessing comparability between innovator and biosimilars. Furthermore, the bioassay can be used to assess the impact of post-translational modifications, such as glycosylation and fucosylation, on the potency of monoclonal antibodies (mAbs).

Many therapeutic monoclonal antibodies (mAbs) include ADCC as part of their mechanism of action (MoA). This makes accurate determination of ADCC activity an essential part of the development and characterization of therapeutic antibodies.
Using the iLite® ADCC Solution enables the researchers to use only one platform for screening a large pool of compounds to establish a mechanism of action/ effect on target.
iLite ADCC cell lines can successfully be used:
When investigating potential drug candidates, it is essential to determine the presence of ADCC activity and if found, quantify the activity. This is an important step in identifying the most potent antibody and in lot release testing. If ADCC has been determined to be the mechanism of action of a biological drug this should be reflected in the potency assay.
iLite cell lines can be successfully used to:
To increase the ADCC activity of therapeutic antibodies, the pharmaceutical industry has structurally improved the Fc region of the antibodies. Point mutations or modifications into the glycosylation profiles of Fc regions have been shown to increase their affinity towards Fc receptors on a range of effector cells.
To evaluate the efficacy of these biotherapeutics, several ADCC methodologies have been developed.
Using iLite® assays, we have shown that deglycosylation of the therapeutic antibodies trastuzumab and rituximab using GlycINATOR enzyme (Genovis) completely abolishes ADCC.
This workflow represents a powerful tool in preclinical development and mode-of-action studies of therapeutic antibody candidates.
With many of the existing patents of monoclonal antibody blockbuster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization are needed to show comparability between innovator and biosimilar and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA, and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo, with ADCC assessments forming an important component.
There are strict requirements to show comparability between innovator drugs and biosimilars. ADCC evaluation studies are an essential part of the comparability profile. It is generally accepted that the innovator and biosimilar will be different due to the complex nature of the production process. However, the regulatory approach is that the compounds must be shown to be sufficiently similar to provide the same clinical outcome when used to treat disease.
Increasing evidence suggests that ADCC plays a significant role in antibody-mediated protection and control of viral infection and several laboratory methods exist for determining the efficacy of antibodies or effector cells in eliciting ADCC.
The iLite ADCC Reporter Bioassay is designed for evaluating ADCC of therapeutic monoclonal antibodies (mAbs) and exhibits greatly reduced variability and offers an easier workflow than traditional ADCC assays.
It is an ideal assay for applications such as potency lot release and antibody screening, as well as for assessing comparability between innovator and biosimilars. Furthermore, the bioassay can be used to assess the impact of post-translational modifications, such as glycosylation and fucosylation, on the potency of monoclonal antibodies (mAbs).

Many therapeutic monoclonal antibodies (mAbs) include ADCC as part of their mechanism of action (MoA). This makes accurate determination of ADCC activity an essential part of the development and characterization of therapeutic antibodies.
Using the iLite® ADCC Solution enables the researchers to use only one platform for screening a large pool of compounds to establish a mechanism of action/ effect on target.
iLite ADCC cell lines can successfully be used:

When investigating potential drug candidates, it is essential to determine the presence of ADCC activity and if found, quantify the activity. This is an important step in identifying the most potent antibody and in lot release testing. If ADCC has been determined to be the mechanism of action of a biological drug this should be reflected in the potency assay.
iLite cell lines can be successfully used to:

To increase the ADCC activity of therapeutic antibodies, the pharmaceutical industry has structurally improved the Fc region of the antibodies. Point mutations or modifications into the glycosylation profiles of Fc regions have been shown to increase their affinity towards Fc receptors on a range of effector cells.
To evaluate the efficacy of these biotherapeutics, several ADCC methodologies have been developed.
Using iLite® assays, we have shown that deglycosylation of the therapeutic antibodies trastuzumab and rituximab using GlycINATOR enzyme (Genovis) completely abolishes ADCC.
This workflow represents a powerful tool in preclinical development and mode-of-action studies of therapeutic antibody candidates.
With many of the existing patents of monoclonal antibody blockbuster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization are needed to show comparability between innovator and biosimilar and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA, and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo, with ADCC assessments forming an important component.
There are strict requirements to show comparability between innovator drugs and biosimilars. ADCC evaluation studies are an essential part of the comparability profile. It is generally accepted that the innovator and biosimilar will be different due to the complex nature of the production process. However, the regulatory approach is that the compounds must be shown to be sufficiently similar to provide the same clinical outcome when used to treat disease.