- Other Products
- CCP
The Svar Complement activity biomarkers give valuable intelligence in several situations where one might suspect that complement activation plays a role in the disorder. The Svar assays for complement activity biomarkers are developed to target the unique neoepitopes only presented at the complement component or complex when activated.
The Svar Complement C4d , Complement TCC, and Complement Factor P assays are flexible and easy to use enzyme immunoassays with ready to use reagents and short incubation times leading to reduced hands-on-time for the user.
The Svar complement activity biomarkers are well suited for studies of complement activation in drug development and medical devices, i.e. biological safety testing and as part of the recommended tests for assessing complement activation according to ISO standard 10993-4 for hemocompatibility testing.
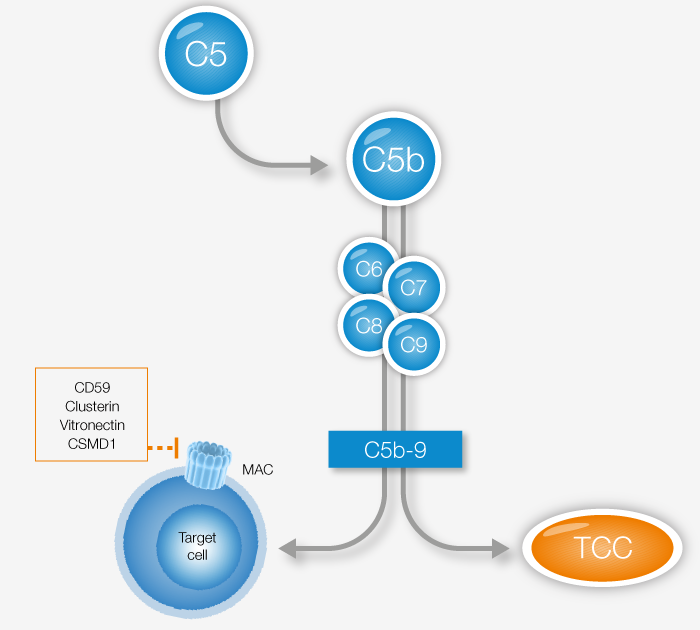
The Terminal Complement Complex, or TCC, is a product of the terminal pathway, thus a result of activation of any of the three complement activation pathways. As a reflection of total in vivo complement activity, increased levels of TCC can be detected in several acute, flaring, and chronic diseases. TCC assays are also among the recommended tests for assessing complement activation according to ISO standard 10993-4 for hemocompatibility testing.
View our TCC product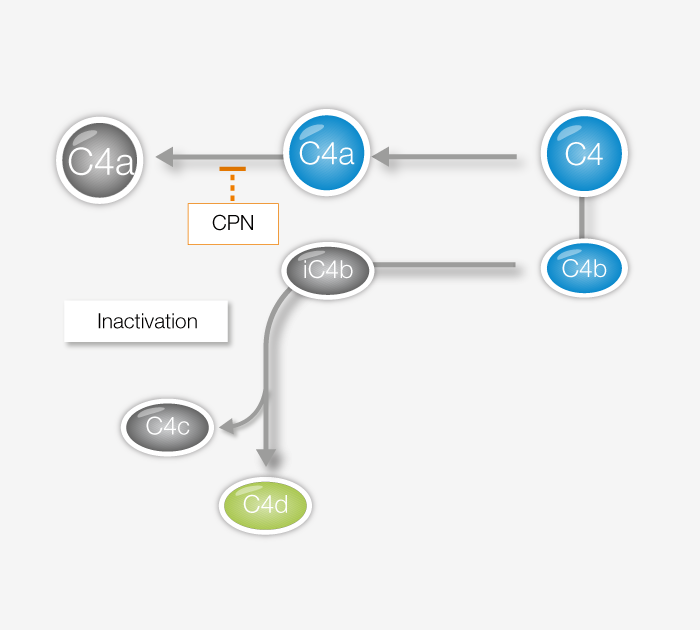
C4d is a marker for complement activity originating from the classical or lectin pathways. It is an established histological biomarker with a strong association to antibody mediated rejection of grafts. Moreover, C4d has increasingly been used as a biomarker for diagnostic and prognostic purposes, in conditions ranging antibody-mediated disease to cancers.
View our C4d product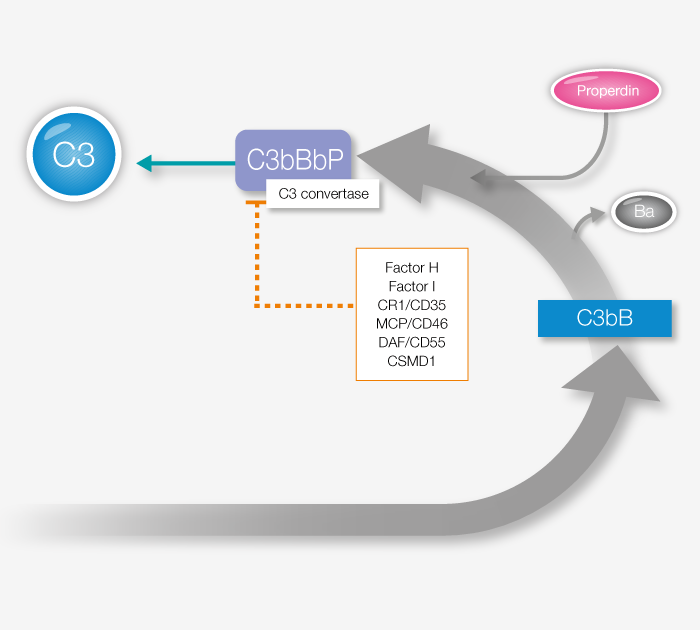
Factor P, also known as Properdin, is the only known positive regulator of alternative pathway complement response and key to ensuring sufficient complement activity. Properdin imbalance or dysfunction has been linked to several health disorders, and assaying its prevalence is a useful tool for a better understanding of complement regulation. In addition to the quantitative Factor P assay linked below, we also offer a functional Factor P assay.
View our Factor P productDuring the last decade, the importance of the complement system has become evident in both clinical medicine and complement therapeutics. This increased interest means a more prominent need for assays to evaluate complement activity.
Activity biomarkers are used to determine whether deficiencies, overactivation or dysregulation in the complement system are causing, or contributing to, a person's disease or condition.
In order to exactly measure such activation, assays for quantification of products formed during activation are required.
Complement activity biomarkers are recommended to predict possible adverse reactions from medical devices such as grafts, stents, hemodialysis and cardiopulmonary bypass but also to biological drugs and nanomedicines.
Complement TCC is part of the recommended tests for assessing complement activation according to ISO standard 10993-4.
Patients are each year are affected by adverse reactions that might be complement activated. These reactions are critical and can lead to death.
Complement activity biomarkers can be used successfully for investigation to rule out if a drug is toxic or if a reaction is due to other related issues such as “infusion reactions” and more specifically complement activation-related pseudoallergy (CARPA). Infusion reactions has been reported to several pharmaceuticals such as biologicals and nanomedicines.