AAV Complement Activation Assay
Adeno-associated virus (AAV)-based gene therapy shows great promise for treating diverse genetic disorders, but high doses have been shown to activate the complement system, risking patient safety and therapeutic efficacy.
Serotype-specific complement activation
Our AAV-associated Complement Activation ELISA is a robust and sensitive serotype-specific assay designed to assess AAV-induced complement activation. Tailoring assessment to individual AAV serotypes allows for informed decisions around vector design and patient selection, ultimately resulting in safer gene therapies.
Key features
Customizable serotype-specific detection
The assay is a 96-well ELISA tailored to wild-type and novel capsid variants, including engineered or chimeric AAVs. This customization ensures accurate profiling of capsid-specific immune responses.
High sensitivity to complement activation
Detects complement activation products deposited in the assay as a result of AAV-triggered complement activation. This detection has been demonstrated even at low antibody titers, reflecting real-world patient variability.
A mechanistic insight
Captures pathway-independent complement activation, mitigating the uncertainties in AAV-mediated immunogenicity and risk of false negatives.
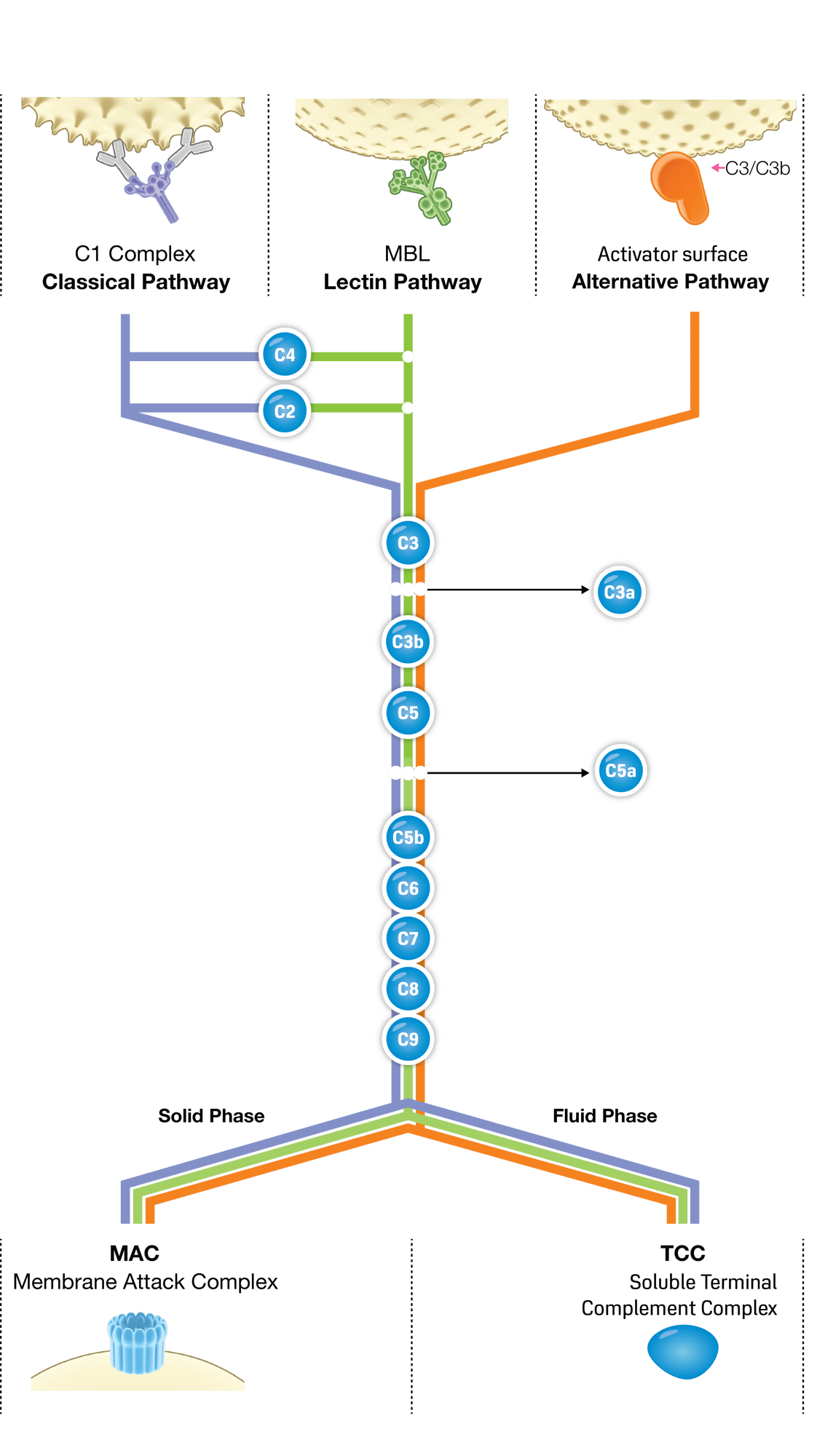
How it works
1. ELISA plate coated with immobilized vector
Wild-type vectors or your custom vector can be used.
2. Capture of Anti-AAV Binders
Anti-AAV antibodies and potential complement activators (e.g., immunoglobulins/C1q, MBL/ficolins, and C3b) are captured from a human serum sample under complement-suppressing conditions.
3. Complement activation
Following a washing step, the serum sample is reapplied in a complement-permissive buffer. If complement-activating binders are present, terminal pathway activity results in TCC deposition.
4. Detection
Detection is performed using an anti-TCC antibody, generating a signal proportional to the level of complement activation.
How to order
This product is currently offered as a custom service, where Svar, based on our long experience in immunogenicity assessment in various pharma settings, is well equipped to solve your needs.
In addition to customized AAV Complement Activation Assay, we offer AAV NAb and TAb assays for a complete AAV immunogenicity solution. These assays and more are also available as a service offering.
Contact us to get started