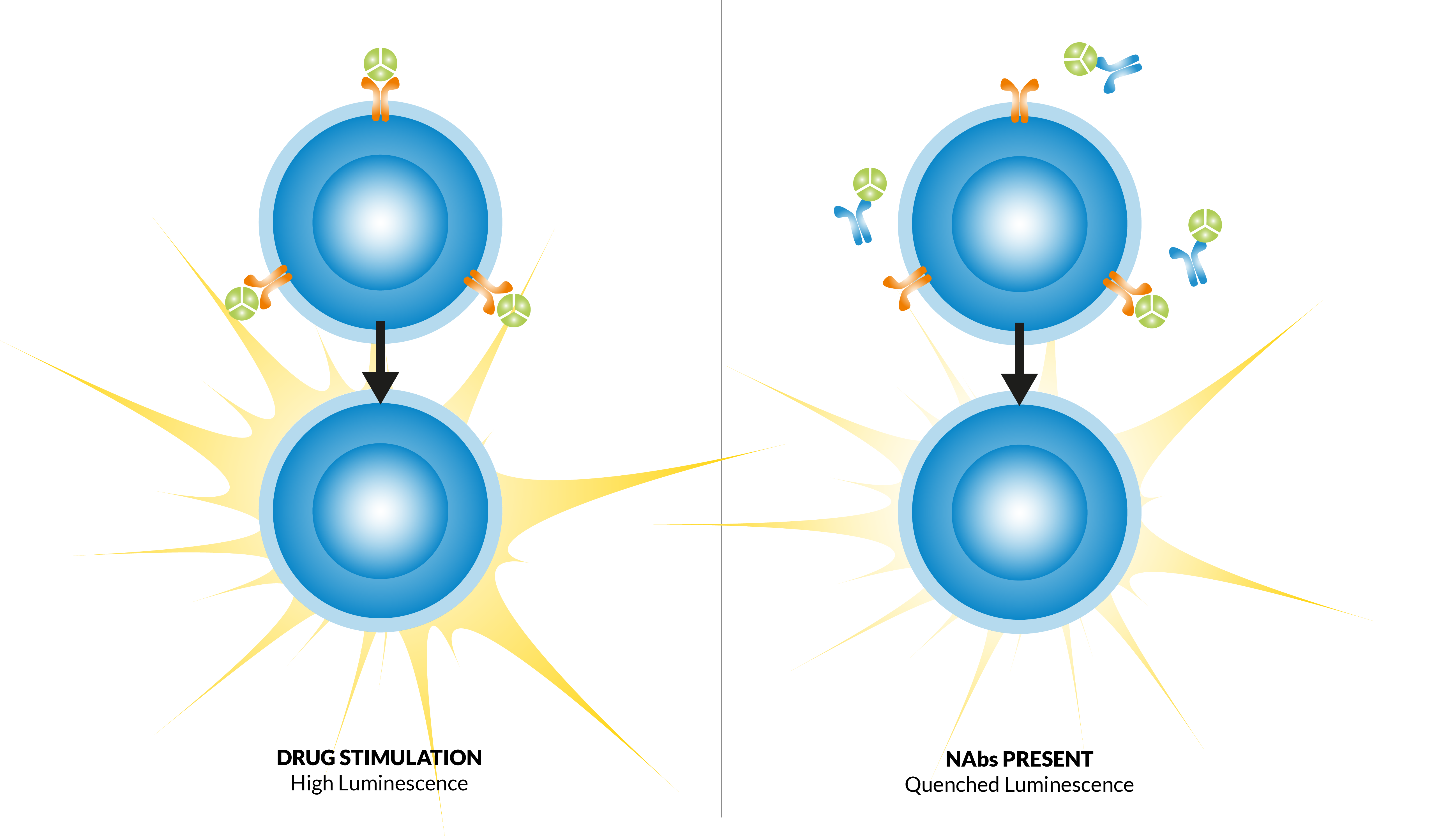
There is a steady rise in the number of proteins being approved for use as therapeutic agents with major therapeutic gains and upsides, but besides the huge benefits that come with the use of these biologics, there is one major limitation that arises when using these therapeutics – formation of anti-drug antibodies (ADAs) and Neutralizing antibodies (Nabs).
The complex nature of these therapeutics and their interactions with various endogenous proteins in the human body give them unique pharmacokinetic and pharmacodynamic (PK/PD) properties and can induce anti-drug antibodies (ADA), some of which act to neutralize the effect of the drug (NAbs). The assessment of such undesirable immunogenicity effects is a key element in biological drug development.
- Other Products
- CCP