- Other Products
- CCP
As part of the Complement Systems, the Classical Pathway (CP) is a natural link between the innate immune system and the acquired immune system.
It has been shown that the CP can be activated by a large number of factors other than antibodies. A wide variety of pathogenic microorganisms efficiently activate the CP after their recognition by antibodies.
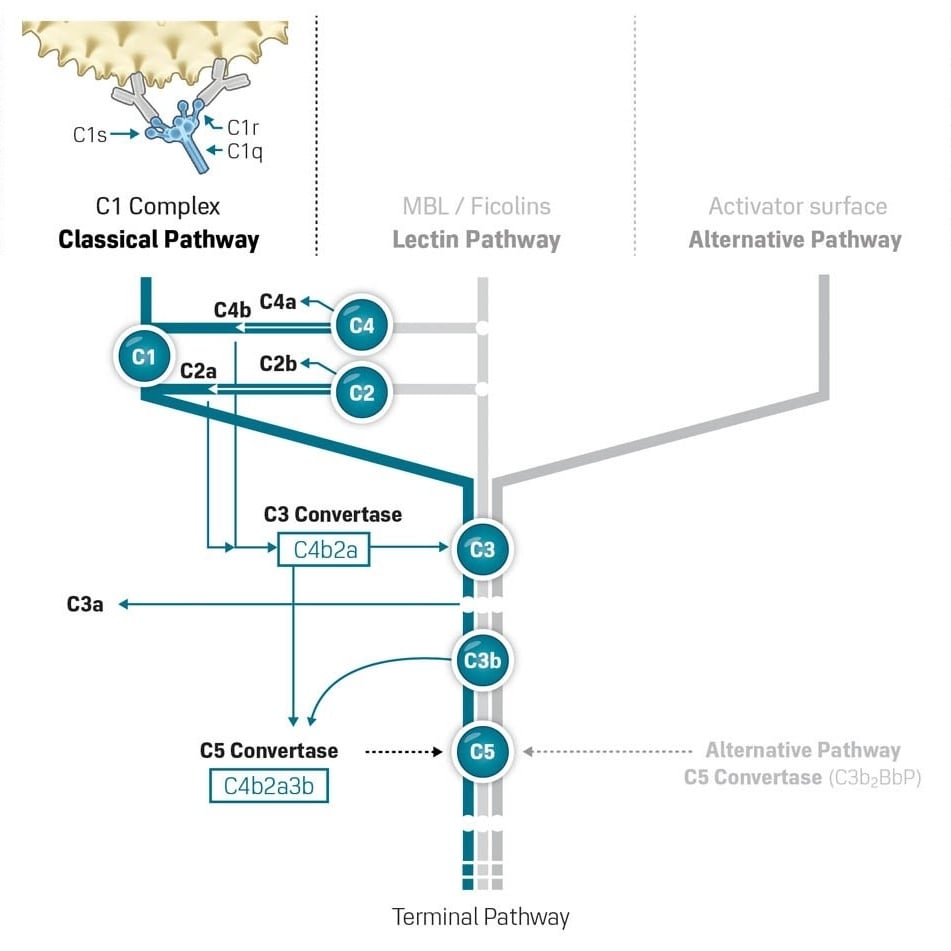
A key event for the activation is the interaction of the serum C1 complex with antibody-antigen complexes or immune aggregates containing IgG or IgM. This is the main activation mechanism of the Classical Pathway.
Binding of antibody induces a conformational change in the C1 complex leading to activation of the C1s and C1r serine protease subunits. The activated C1 complex cleaves C4 resulting in a reactive C4b which covalently binds to proteins or polysaccharides at the surface in close proximity of the C1. The bound C4b binds C2 and which renders C2 available for proteolysis by C1. The formed complex C4b2b (formerly called C4b2a), is the CP C3 convertase. This C3 convertase is responsible for the important cleavage of C3 to C3a and C3b. It is regulated in part by the binding of membrane bound DAF which increase the decay of the C3 convertase. Other important regulatory factors are Factor I and Factor H. Native C3b is able to covalently bind to surfaces in close vicinity of the C3 convertase, resulting in forming the CP C5 convertase (C4b2a3b).
In the processes discussed above fragments from C3 and C4, i.e. C3a and C4a are released into the circulation. These components both acts as anaphylatoxins binding to receptors on the surface of mast cells initiating release of vasoactive amines. They may also bind to receptors on neutrophils stimulating release of lysosomal enzymes. Both fragments contribute to inflammation although C3a is the most efficient.
The formation of C5 convertase is the first step of the lytic part of the complement pathways. This convertase, either formed by components of the classical/lectin pathway (C4b2a3b) or the alternative pathway (C3b2BbP), cleaves C5 to C5a and C5b. The C5b is the first component of the self-assembly of Membrane Attack Complex (MAC) or its soluble counterpart soluble Terminal Complement Complex (sTCC).