- Other Products
- CCP
Cell-based assays is a versatile tool for the whole drug development process and there are many reason for conducting cell based assays.
As many of the biological drugs that exists, or are currently under development, have multiple functional domains that interact with several different molecules in order to function appropriately, different tests and validations of the function, efficiency and safety must be performed before the end product is released.
In a regulatory setting, cell based assays are commonly used for cytotoxicity testing, to determine the biological activity (potency) of drug product and drug substance, to determine the mechanism of action (MOA), early stage proof of principal studies, and in immunogenicity studies to determine if antibodies produced by the patient are neutralizing the drug product.
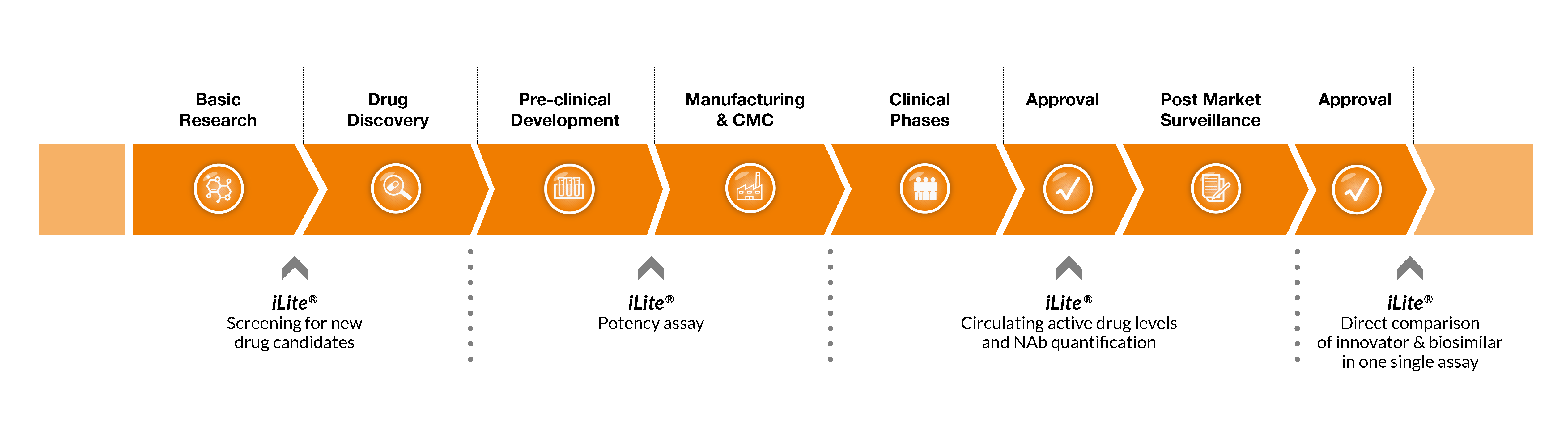
During the basic research phase scientists are working to gain an in-depth understanding of a disease, the biological mechanism behind it and to identify potential compounds that might be targeted with a new drug.
Drug discovery is a wide definition of the work that takes place after the target has been identified. This work can include many types of projects but typically the focus is on target validation and lead optimization.
Once a drug candidate is selected, preclinical studies are performed to evaluate its safety, efficacy, and potential toxicity. Pre-clinical studies help the researchers to design the studies for the clinical trials and safety evaluation criteria are identified.
The chemistry, manufacturing and controls (CMC) process is pivotal in ensuring that drugs and treatments being manufactured are safe, effective and of a high quality for patients.
During the Clinical trials a drug is tested in humans for the first time. First in a population of healthy volunteers (phase I), then in a small patient population (phase II) and finally in a larger patient population (phase III).
Postmarketing surveillance refers to the process of monitoring the safety of drugs once they reach the market, after the successful completion of clinical trials. The primary purpose is to identify previously unrecognized adverse effects as well as positive effects.
The iLite functional reporter bioassays are used to characterize and develop novel therapeutics – from high throughput screening, through CMC lot release testing, to determination of immunogenicity.
It is a versatile tool with applications throughout the drug continuum due to its clever engineering and use in accurate functional assessments.
At an early stage in the development process, thousands of compounds need to be narrowed down to a small number of the most promising candidates.
In order to identify the best candidates, you should use an assay that as closely as possible reflects the complex processes of the cell, where different proteins or pathways are induced or inhibited depending on the circumstances. It is not sufficient to just determine whether or not the compound binds to its intended target.
Our cell-based iLite assays offer this functionality. In addition to finding out if the candidate drug binds to it target, you get additional information that can help you determine if the compound functions according to its intended mechanism of action.
Determining the potency of a candidate is one of the most essential parts of drug development.
Based on the regulatory guidelines, the potency of the drug candidate often needs to be addressed by a cell-based assay to achieve the biological relevance required. Potency assays also need to be highly reproducible.
The iLite system fits both criteria and can be used for measuring the drug candidate’s potency for Quality Control and as a batch release assay. Typically, it has to be established that the drug gives the same potency (effectiveness) for each batch when tested in a biologically relevant setting,
iLite cell lines can be successfully used to:
Immunogenicity is an immune response against a therapeutic antigen causing that medication to lose its effectiveness over time and potentially resulting in serious illness. Clearly, immunogenicity is an important factor to consider as manufacturers develop new protein therapeutics.
Because drug development becomes increasingly costly as it moves downstream, it is important to anticipate immunogenicity as far upstream as possible in the drug discovery process.
Customers assessing immunogenicity need an Anti-Drug Antibody (ADA) assay and a NAb assay. While ADAs are most easily assessed by a regular ligand binding assay, detection of NAbs most often requires a functional cell-based assay.
Requirements for NAb assays are:
With many of the existing patents of monoclonal antibody block buster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization is needed to show comparability between innovator and biosimilars and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo. Here, iLite Functional Bioassay Profiles could be of great value, when assessing and comparing the response of the compounds.
At an early stage in the development process, thousands of compounds need to be narrowed down to a small number of the most promising candidates.
In order to identify the best candidates, you should use an assay that as closely as possible reflects the complex processes of the cell, where different proteins or pathways are induced or inhibited depending on the circumstances. It is not sufficient to just determine whether or not the compound binds to its intended target.
Our cell-based iLite assays offer this functionality. In addition to finding out if the candidate drug binds to it target, you get additional information that can help you determine if the compound functions according to its intended mechanism of action.
Determining the potency of a candidate is one of the most essential parts of drug development.
Based on the regulatory guidelines, the potency of the drug candidate often needs to be addressed by a cell-based assay to achieve the biological relevance required. Potency assays also need to be highly reproducible.
The iLite system fits both criteria and can be used for measuring the drug candidate’s potency for Quality Control and as a batch release assay. Typically, it has to be established that the drug gives the same potency (effectiveness) for each batch when tested in a biologically relevant setting,
iLite cell lines can be successfully used to:
Immunogenicity is an immune response against a therapeutic antigen causing that medication to lose its effectiveness over time and potentially resulting in serious illness. Clearly, immunogenicity is an important factor to consider as manufacturers develop new protein therapeutics.
Because drug development becomes increasingly costly as it moves downstream, it is important to anticipate immunogenicity as far upstream as possible in the drug discovery process.
Customers assessing immunogenicity need an Anti-Drug Antibody (ADA) assay and a NAb assay. While ADAs are most easily assessed by a regular ligand binding assay, detection of NAbs most often requires a functional cell-based assay.
Requirements for NAb assays are:
With many of the existing patents of monoclonal antibody block buster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization is needed to show comparability between innovator and biosimilars and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo. Here, iLite Functional Bioassay Profiles could be of great value, when assessing and comparing the response of the compounds.
Increasing evidence suggests that antibody-dependent cellular cytotoxicity (ADCC) plays a significant role in antibody-mediated protection and control of viral infection and several laboratory methods exist for determining the efficacy of antibodies or effector cells in eliciting ADCC.
The iLite ADCC Reporter Bioassay is designed for evaluating ADCC of therapeutic monoclonal antibodies (mAbs) and exhibits greatly reduced variability and offers an easier workflow than traditional ADCC assays. It is an ideal assay for applications such as potency lot release and antibody screening, as well as for assessing comparability between innovator and biosimilars. Furthermore, the bioassay can be used to assess the impact of post-translational modifications, such as glycosylation and fucosylation, on the potency of monoclonal antibodies (mAbs).
Many therapeutic monoclonal antibodies (mAbs) include ADCC as part of their mechanism of action (MoA). This makes accurate determination of ADCC activity an essential part of the development and characterization of therapeutic antibodies.
Using the iLite ADCC Solution enables the researchers to use only one platform for screening a large pool of compounds to establish a mechanism of action/ effect on target.
iLite ADCC cell lines can successfully be used:
Potency for therapeutic antibodies
When investigating potential drug candidates, it is essential to determine the presence of ADCC activity and if found, quantify the activity. This is an important step in identifying the most potent antibody and in lot release testing. If ADCC has been determined to be the mechanism of action of a biological drug this should be reflected in the potency assay.
iLite cell lines can be successfully used to:
To increase the ADCC activity of therapeutic antibodies, the pharmaceutical industry has structurally improved the Fc region of the antibodies. Point mutations or modifications into the glycosylation profiles of Fc regions have been shown to increase their affinity towards Fc receptors on a range of effector cells. To evaluate the efficacy of these biotherapeutics, several ADCC methodologies have been developed.
Using iLite assays, we have shown that deglycosylation of the therapeutic antibodies trastuzumab and rituximab using GlycINATOR enzyme (Genovis) completely abolishes ADCC. This workflow represents a powerful tool in preclinical development and mode-of-action studies of therapeutic antibody candidates.
With many of the existing patents of monoclonal antibody block buster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization is needed to show comparability between innovator and biosimilar and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo, with ADCC assessments forming an important component.
There are strict requirements to show comparability between innovator drugs and biosimilars. ADCC evaluation studies are an essential part of the comparability profile. It is generally accepted that the innovator and biosimilar will be different due to the complex nature of the production process. However, the regulatory approach is that the compounds must be shown to be sufficiently similar to provide the same clinical outcome when used to treat disease.
Many therapeutic monoclonal antibodies (mAbs) include ADCC as part of their mechanism of action (MoA). This makes accurate determination of ADCC activity an essential part of the development and characterization of therapeutic antibodies.
Using the iLite ADCC Solution enables the researchers to use only one platform for screening a large pool of compounds to establish a mechanism of action/ effect on target.
iLite ADCC cell lines can successfully be used:
Potency for therapeutic antibodies
When investigating potential drug candidates, it is essential to determine the presence of ADCC activity and if found, quantify the activity. This is an important step in identifying the most potent antibody and in lot release testing. If ADCC has been determined to be the mechanism of action of a biological drug this should be reflected in the potency assay.
iLite cell lines can be successfully used to:
To increase the ADCC activity of therapeutic antibodies, the pharmaceutical industry has structurally improved the Fc region of the antibodies. Point mutations or modifications into the glycosylation profiles of Fc regions have been shown to increase their affinity towards Fc receptors on a range of effector cells. To evaluate the efficacy of these biotherapeutics, several ADCC methodologies have been developed.
Using iLite assays, we have shown that deglycosylation of the therapeutic antibodies trastuzumab and rituximab using GlycINATOR enzyme (Genovis) completely abolishes ADCC. This workflow represents a powerful tool in preclinical development and mode-of-action studies of therapeutic antibody candidates.
With many of the existing patents of monoclonal antibody block buster drugs set to expire in the next few years, the development of biologic therapeutics similar to the original drug (biosimilars) has become increasingly important.
However, extensive requirements for analytical characterization is needed to show comparability between innovator and biosimilar and it must be proven that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency”. This is the biosimilar development approach from the EMA, FDA and WHO.
The most critical evaluation is that of biological function, through assays that replicate the likely mechanism of action in vivo, with ADCC assessments forming an important component.
There are strict requirements to show comparability between innovator drugs and biosimilars. ADCC evaluation studies are an essential part of the comparability profile. It is generally accepted that the innovator and biosimilar will be different due to the complex nature of the production process. However, the regulatory approach is that the compounds must be shown to be sufficiently similar to provide the same clinical outcome when used to treat disease.
CRO SERVICES
Through use of the iLite® technology for our cell-based Neutralizing Antibody bioassays, our bioanalytical services can offer a full characterization of NAbs.
Learn about Neutralizing Antibody (NAb) assays and why cell-based bioassays are preferred over ligand-binding assays.
WIESLAB DIAGNOSTIC SERVICES
To measure the biological activity of a drug, Wieslab Diagnostic Services use the iLite technology which gives a high therapeutic significance. If the test shows that the patient has low concentrations of the TNF-α inhibitors and/or has developed NAbs, it is both clinically and economically correct to adjust the treatment regime.