- Other Products
- CCP
Are you looking for reliable immunogenicity testing? This blog post illustrates how advanced tools and expert bioanalytical services can assist you in your immunogenicity assessment.
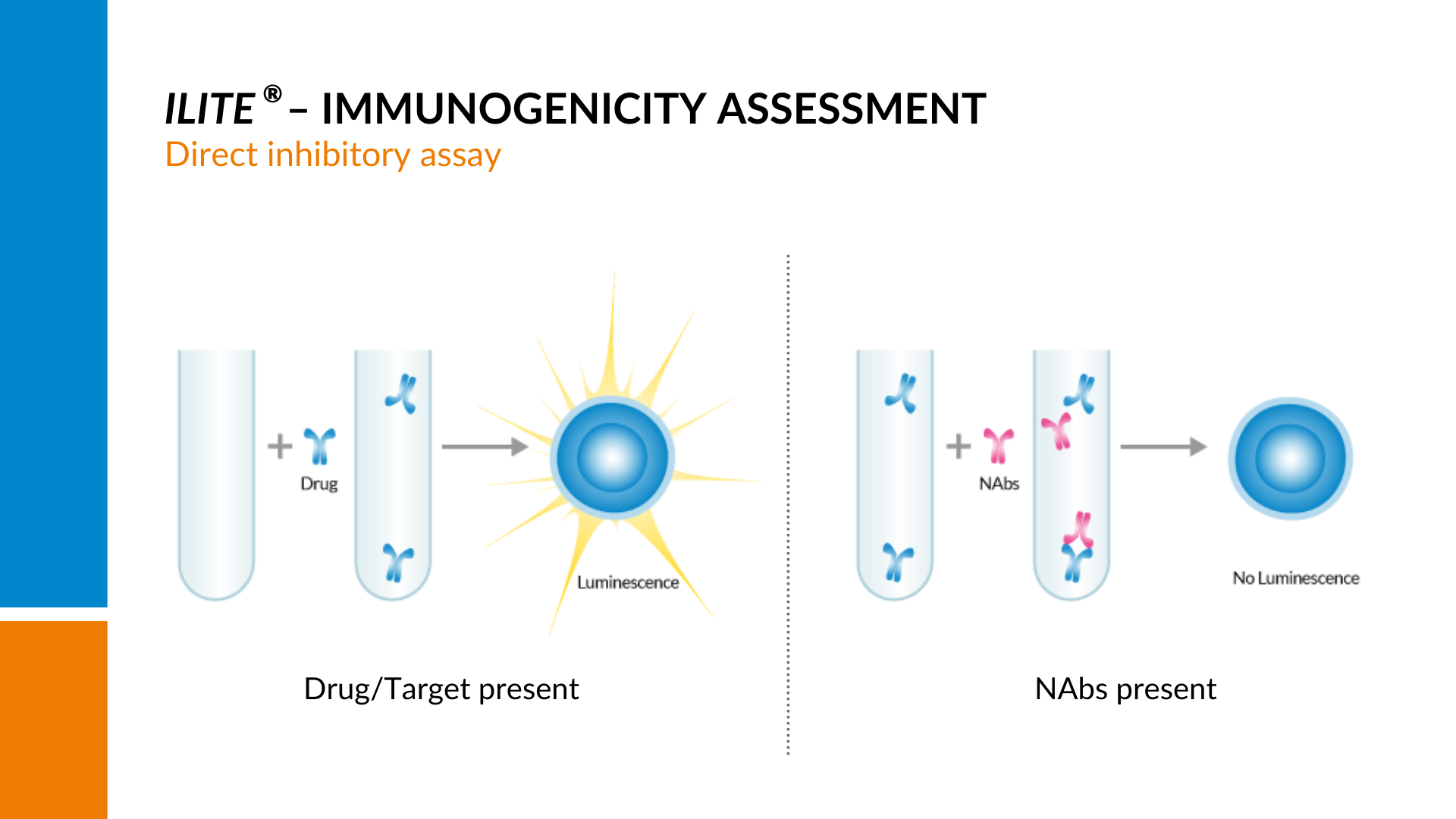
Our iLite® Immunogenicity Assessment provides an advanced direct inhibitory assay to measure neutralizing antibodies (NAbs) with high specificity and sensitivity.
.jpg)
.jpg)
Are you looking for reliable immunogenicity testing? This blog post illustrates how advanced tools and expert bioanalytical services can assist you in your immunogenicity assessment.
Our iLite® Immunogenicity Assessment provides an advanced direct inhibitory assay to measure neutralizing antibodies (NAbs) with high specificity and sensitivity.
The standard approach , when determining the immunogenicity profile of a drug compound, is to measure ADAs and NAbs using a multi-tiered approach.
1. Screening Assay: Detects ADAs that bind to the biological drug, usually performed using Ligand Binding Assay (LBA) techniques such as ELISA.
3. Characterization of Confirmed ADA Positive Samples: A functional assay, often a cell-based assay, is required to determine the binding type and clinical significance of ADAs.
By combining ligand binding assays immunogenicity knowledge with cell-based assays, we achieve a full characterization of ADAs and NAbs.
This means more information and a better understanding of the immune system compounds to develop your risk mitigation strategy — the more informed choices you can make, the more likely your projects will be successful.
The iLite technology is a cleverly engineered cell-based assay system with a dual reporter gene readout. It offers the ease of use and robustness of a ligand-binding assay and can be developed for virtually any pharmaceutical target.
It allows an easy, rapid, and accurate test format for measuring and quantifying drug activity and immunogenicity.
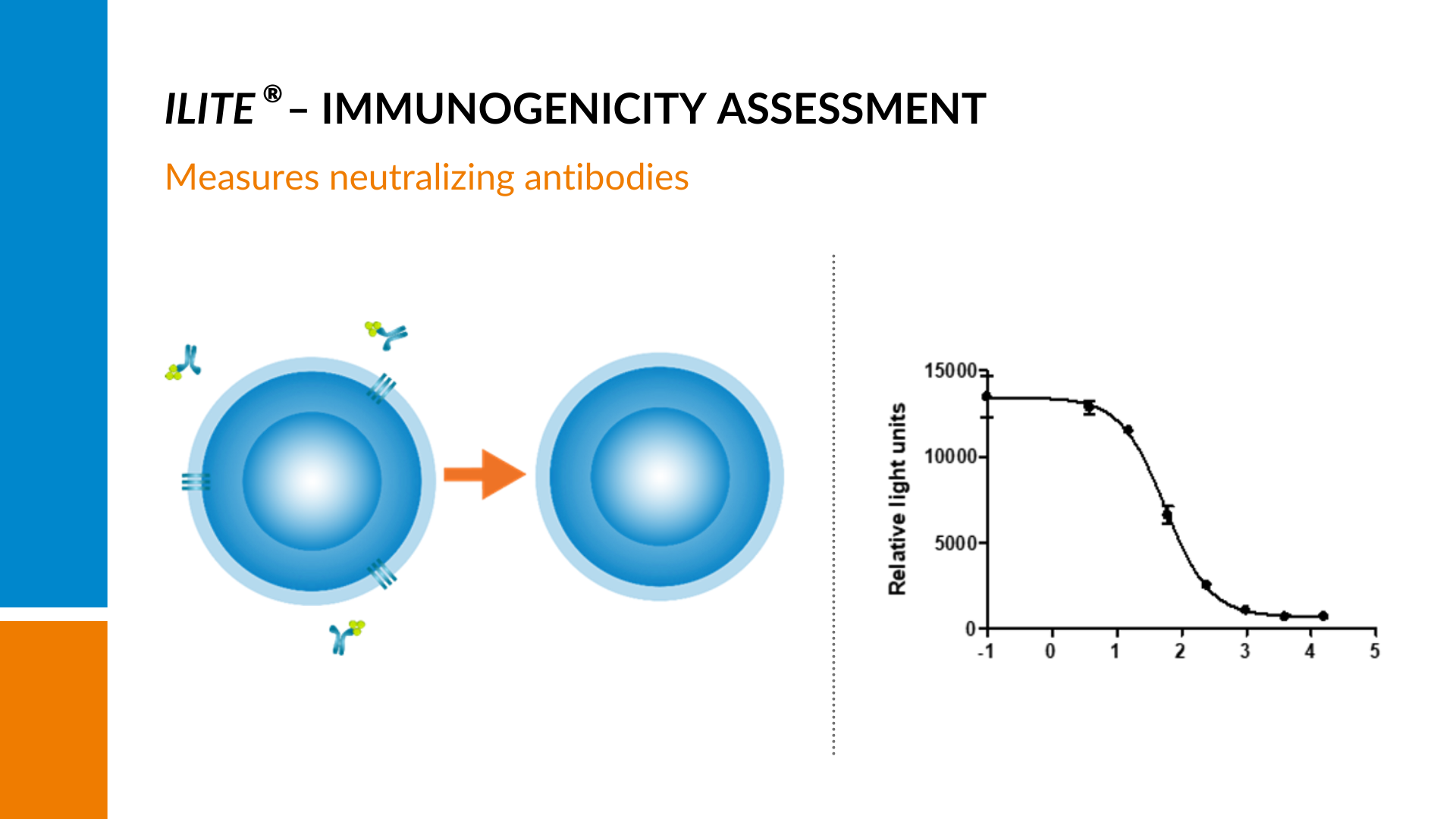
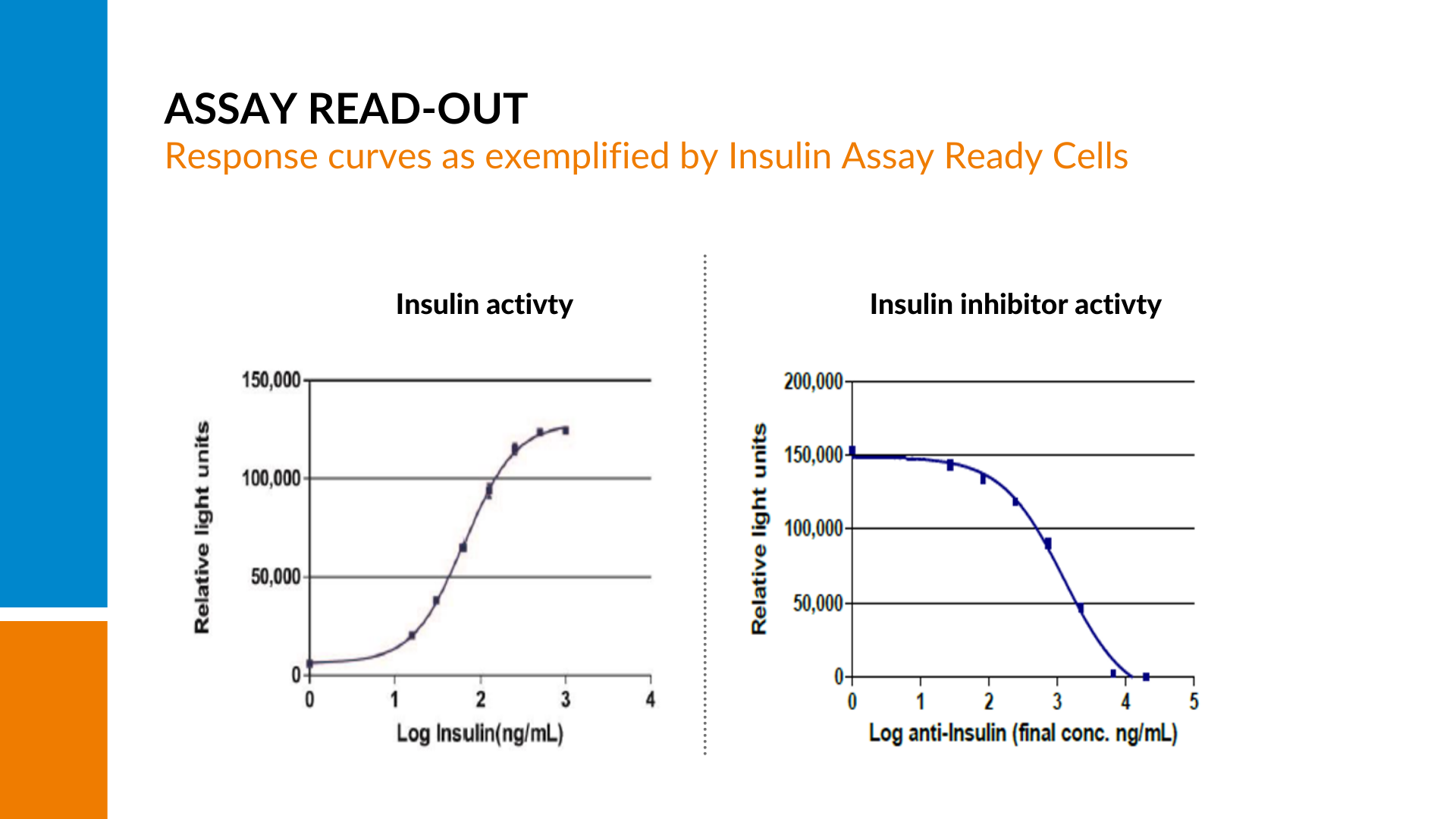
When iLite cells are stimulated with their specific target, a measurable dose-dependent signal is generated.
This signal is indicative of the cell’s activity in response to the stimulating agent, typically a drug or ligand. However, If there are neutralizing antibodies present in the sample, the amount of free drug to stimulate the cells will be reduced, resulting in a quenching of the signal and a decline in the curve with increasing amounts of neutralizing antibodies present.