- Other Products
- CCP
Over the last few years, it has become clear that having a better understanding of the complex mechanisms of the Complement System and all its physiological effects is paramount to providing access to better care and health outcomes for those suffering from complement-mediated rare diseases. Increasing this understanding is also essential for developing better therapeutics for these patients.
Today, we join the rare disease community in their mission to raise awareness for rare diseases. And in doing so, we will continue to work towards providing innovative solutions to support the development of better treatments.
.png)
.png)
Over the last few years, it has become clear that having a better understanding of the complex mechanisms of the Complement System and all its physiological effects is paramount to providing access to better care and health outcomes for those suffering from complement-mediated rare diseases. Increasing this understanding is also essential for developing better therapeutics for these patients.
Today, we join the rare disease community in their mission to raise awareness for rare diseases. And in doing so, we will continue to work towards providing innovative solutions to support the development of better treatments.
Million people are living with a rare disease1
of the worldwide population is currently affected by a rare disease1
rare diseases have been identified1, within these are included deficiencies or overactivation of the complement system
of rare diseases do not have an FDA-approved therapy2, there is an unmet therapeutic need
The Complement System is a critical component of the innate immune system that helps us respond to invading microorganisms, clears damaged cells, initiates a cellular immune response, and maintains optimal health. Certain genetic and acquired factors can cause the system to become unbalanced, leading to more destruction than defense. Click to read more about the Complement System.
The alternative pathway (AP) is one of the three activation pathways of the complement system. A part of this pathway overlaps with the amplification loop of the Complement System. The amplification loop is an integral part of the expansion of the initial response.
The amplification loop comprises a range of components, like Factor B and Factor D, and modulators crucial for its functioning and ability to regulate the overall cascade. These include down regulators such as DAF (CD55), Factor H, and Factor I, as well as the only known up regulator, properdin (Factor P), that serves as a stabilizer for the convertases of the alternative pathway. The alternative pathway and the amplification loop are central to ensuring a prompt response; however, if overactivated, it can potentially result in excessive inflammation and tissue damage.
Complement-mediated diseases are disorders caused by a lack of or uncontrolled activation of the Complement System. There are many rare conditions and rare diseases connected with complement component deficiencies, and they are associated with the development of autoimmune conditions2 and thrombotic disorders such as hereditary angioedema (HAE)3.
Given the key role of the alternative pathway in amplifying the Complement System response, the uncontrolled activation or dysregulation of the alternative pathway has been implicated in several rare diseases, including atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy (C3G). The dysregulation can also contribute to developing paroxysmal nocturnal hemoglobinuria (PNH), another rare condition.
Recently it has been recognized that the Complement System plays a role in rare diseases that initially were not thought to be complement-mediated, such as Myasthenia gravis4 and Sickle-cell disease 5–7.
Rare diseases affect millions of people worldwide; they are complex, often not well understood, and therefore difficult to treat. Although current therapies have demonstrated benefits, they come with limitations, and there are still unmet needs to be addressed to be able to treat many patients. For example, the Complement System, in many cases, remains overactive despite treatment with C5 or C3 inhibitors8. An additional key limitation of these inhibitors is the increased risk of infections resulting from blocking the entire complement cascade9.
We recognize that treatments are needed to improve the quality of life for those living with complement-mediated rare diseases. Recent advances in understanding the Complement System have opened possibilities for emerging and next-generation complement therapeutics targeting specific components of the alternative pathway and the amplification loop.
This is evident from the number of investigational therapies targeting Factor B, factor D, and properdin being studied in clinical trials 10–18.
Targeting the alternative pathway and amplification loop aim to provide better regulation of the complement system rather than complement inhibition, potentially reducing side effects and improving the safety and efficacy of treatments. Additionally, the findings of Complement System involvement in other rare diseases is exciting since it opens up to new complement-targeted therapeutic strategies in new indications.
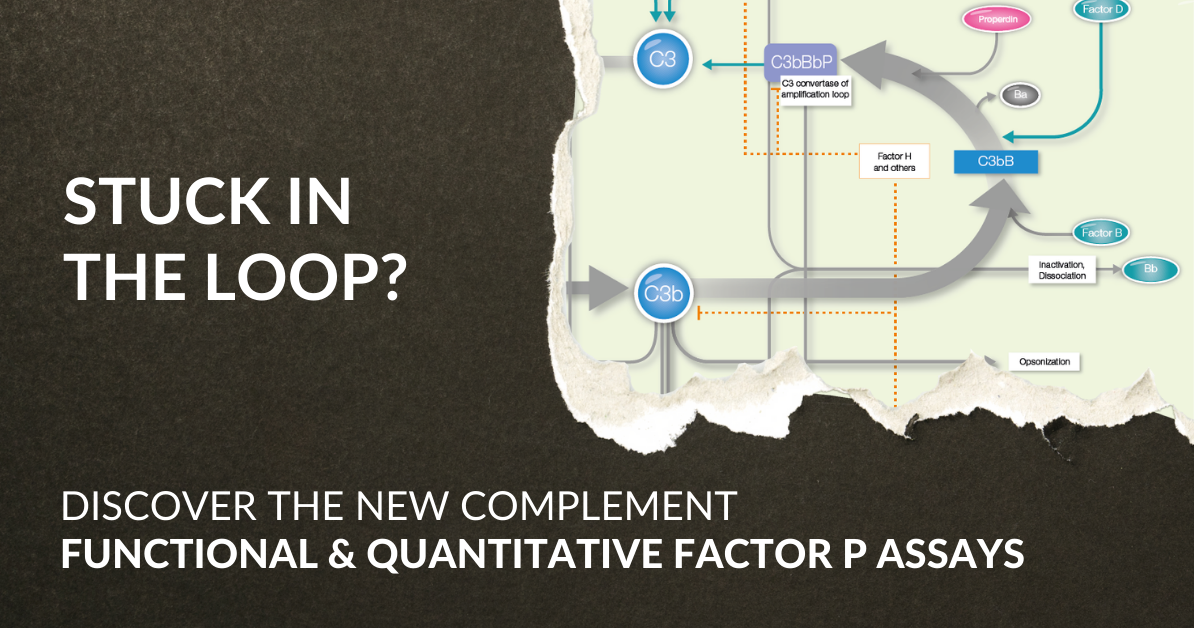
Factor P functional assay, the latest addition to our Complement product range!
Together the quantitative and functional features give you the possibility that, apart from measuring the levels of properdin and determining its functionality, to easily adapt for more in-depth studies, granting a closer look at the amplification loop.
Click here to learn more about our new Factor P functional & quantitative assays.
Our team of experts has the experience and skills to help develop a successful complement therapy. We have developed a full range of functional assays and biomarker assays to allow the examination of the complement system in detail, for research or diagnostics.
We also provide cell-based reporter gene analysis through our iLite® assays custom cell-based services to full fill the requirements of complement drug development.
Our team is committed to providing you with the highest quality assays to aid in your research. We understand the importance of the complement system and are dedicated to helping you develop successful therapies for rare diseases. With our experience, we can help you craft therapies that are precise, reliable, and safe.
Talk to us today to learn more about how we can support your complement needs.
Contact us!
1. S, N. W. et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. European journal of human genetics : EJHG 28, (2020).
2. National Organization for Rare Disorders (NORD®) Orphan Drugs in the United States: An Examination of Patents and Orphan Drug ExclusivityReport-2021_FNL-1.pdf.
3. Mayilyan, K. R. Complement genetics, deficiencies, and disease associations. Protein Cell 3, 487–496 (2012).
4. Schröder-Braunstein, J. & Kirschfink, M. Complement deficiencies and dysregulation: Pathophysiological consequences, modern analysis, and clinical management. Mol Immunol 114, 299–311 (2019).
5. Albazli, K., Kaminski, H. J. & Howard, J. F. Complement Inhibitor Therapy for Myasthenia Gravis. Front Immunol 11, 917 (2020).
6. Tampaki, A., Gavriilaki, E., Varelas, C., Anagnostopoulos, A. & Vlachaki, E. Complement in sickle cell disease and targeted therapy: I know one thing, that I know nothing. Blood Rev 48, 100805 (2021).
7.Varelas, C. et al. Complement in Sickle Cell Disease: Are We Ready for Prime Time? J Blood Med 12, 177–187 (2021).
8. Merle, N. S. et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 3, e96910.
Harder, M. J. et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood 129, 970–980 (2017).
9. McNamara, L. A. et al. High Risk for Invasive Meningococcal Disease Among Patients Receiving Eculizumab (Soliris) Despite Receipt of Meningococcal Vaccine. 66, (2017).
10. Novartis Pharmaceuticals. A Multicenter, Single-arm, Open Label Trial to Evaluate Efficacy and Safety of Oral, Twice Daily LNP023 in Adult aHUS Patients Who Are Naive to Complement Inhibitor Therapy. https://clinicaltrials.gov/ct2/show/NCT04889430 (2023).
11. Alexion Pharmaceuticals. A Phase 2a Proof-of-Mechanism, Open-Label Study to Determine the Effect of ACH-0144471 on C3 Levels in Participants With Low C3 Levels Due to Either C3 Glomerulopathy (C3G) or Immune-Complex Membranoproliferative Glomerulonephritis (IC-MPGN). https://clinicaltrials.gov/ct2/show/NCT03124368 (2021).
12. Alexion Pharmaceuticals. A Phase 2, Proof-of-Concept, Randomized, Double-Blinded, Placebo-Controlled Study of ACH-0144471 Treatment for 6 Months in Patients With C3 Glomerulopathy (C3G), With an Open-label Extension. https://clinicaltrials.gov/ct2/show/NCT03369236 (2022).
13. Novartis Pharmaceuticals. A Multicenter, Randomized, Double-blind, Parallel Group, Placebo-controlled Study to Evaluate the Efficacy and Safety of Iptacopan (LNP023) in Complement 3 Glomerulopathy. https://clinicaltrials.gov/ct2/show/NCT04817618 (2022).
14. Alexion Pharmaceuticals. A Phase 2 Open-label Study of ACH-0144471 in Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH) Who Have an Inadequate Response to Eculizumab Monotherapy. https://clinicaltrials.gov/ct2/show/NCT03472885 (2023).
15. Alexion Pharmaceuticals. A Phase 2 Open-Label Proof of Concept Study to Assess the Efficacy, Safety, and Pharmacokinetics of the Oral Factor D (FD) Inhibitor ALXN2050 (ACH-0145228) in Paroxysmal Nocturnal Hemoglobinuria (PNH) Patients as Monotherapy. https://clinicaltrials.gov/ct2/show/NCT04170023 (2023).
16. Novartis Pharmaceuticals. An Open Label, Single Arm, Multiple Dose Study to Assess Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of LNP023 When Administered in Addition to Standard of Care (SoC) in Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH) With Signs of Active Hemolysis. https://clinicaltrials.gov/ct2/show/NCT03439839 (2022).
17. Novartis Pharmaceuticals. A Randomized, Multicenter, Active-comparator Controlled, Open-label Trial to Evaluate Efficacy and Safety of Oral, Twice Daily LNP023 in Adult Patients With PNH and Residual Anemia, Despite Treatment With an Intravenous Anti-C5 Antibody. https://clinicaltrials.gov/ct2/show/NCT04558918 (2023).
18. Novartis Pharmaceuticals. An Open-label Proof of Concept Study to Assess the Efficacy, Safety and Pharmacokinetics of LFG316, an Anti-C5 Monoclonal Antibody in Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH). https://clinicaltrials.gov/ct2/show/NCT02534909
WANT TO KNOW MORE?
For more information about rare diseases, and how you can contribute to Rare Disease Day, please go to: https://rarediseases.org/and https://www.rarediseaseday.org/