- Other Products
- CCP
Since its discovery in 2019, SARS-CoV-2, the virus that causes covid-19, has been found in many variants. In September 2020, a variant labeled B.1.1.7 was isolated in the UK and quickly became the prevailing lineage worldwide. It is assumed to be around 50% more transmissible than other variants.
As we now know, some covid-19 patients develop acute respiratory failure which can be life threatening. They often require treatment with antipyretics, supplemental oxygen, or mechanical ventilation.

Since its discovery in 2019, SARS-CoV-2, the virus that causes covid-19, has been found in many variants. In September 2020, a variant labeled B.1.1.7 was isolated in the UK and quickly became the prevailing lineage worldwide. It is assumed to be around 50% more transmissible than other variants.
As we now know, some covid-19 patients develop acute respiratory failure which can be life threatening. They often require treatment with antipyretics, supplemental oxygen, or mechanical ventilation.
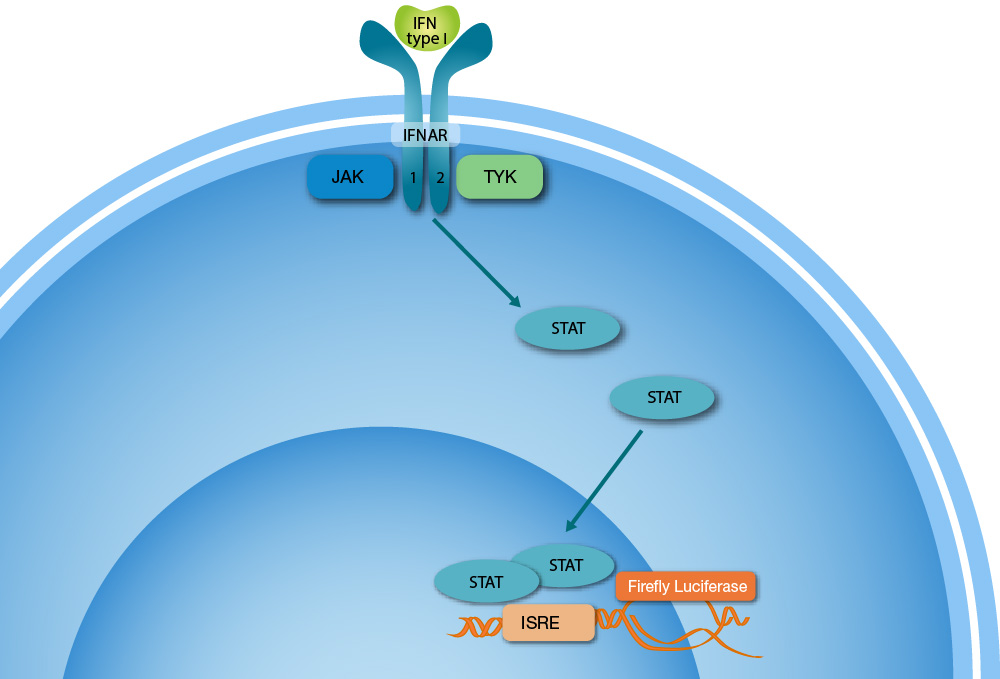
Studies have shown that type I interferon (IFN), which plays an important role in the innate immune response, is lower in patients with severe symptoms compared to patients with mild or moderate symptoms. In addition, approximately 10 % of patients with life-threatening covid-19 pneumonia have neutralizing antibodies against Type I IFNs and undetectable circulating levels during acute disease.
In a recent paper, Thorne and colleagues found that the B.1.1.7 variant of the virus more effectively antagonizes host innate immune responses through upregulation of specific sub-genomic RNA and increased protein expression of key innate immune antagonists. In addition, B.1.1.7 infection was found to induce less IFNβ and was less sensitive to its effects. The authors propose that the virus has evolved beyond the Spike coding region to antagonize host innate immune responses more effectively.
This research clearly indicates that there is a need to accurately monitor both the activity of circulating levels of type I IFNs and auto-antibodies to type I IFNs. This requires a highly sensitive method of detection, like Svar Life Science’s iLite® reporter-gene technology that can detect as little as 1.0 IU/ml of type I IFN (specific activity 2 x 108 IU/mg), as well as anti-IFN autoantibodies with high accuracy and ease of use.
Due to the functional nature of iLite® Type I IFN Assay Ready Cells, the cells can be used to either measure the functional activity of type I IFN or for the detection of neutralizing antibodies (NAbs) against type I IFN – all in the same assay.