- Other Products
- CCP
Biomarker research can be influential across all phases of drug development, from drug discovery and preclinical evaluations to clinical development and post-marketing studies. Biomarkers are important tools for informed decision-making and can include everything from proteins, peptides, cells, histological data, to genetic markers, such as mRNA, SNPs, and microRNA.
The World Health Organization (WHO) in coordination with two other organizations, has defined a biomarker as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”.
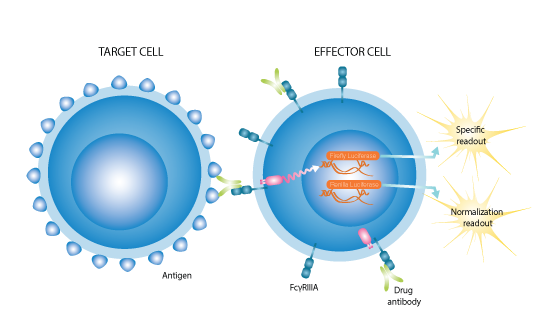

Biomarker research can be influential across all phases of drug development, from drug discovery and preclinical evaluations to clinical development and post-marketing studies. Biomarkers are important tools for informed decision-making and can include everything from proteins, peptides, cells, histological data, to genetic markers, such as mRNA, SNPs, and microRNA.
The World Health Organization (WHO) in coordination with two other organizations, has defined a biomarker as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”.
Biomarkers can be divided into the following categories:
In clinical trials, biomarkers can be used to identify subgroups in the stratification of study subjects, as well as for exploratory purposes. Other uses of biomarkers include:
Some biomarkers are used as substitutes or “surrogates” for safety or efficacy endpoints in clinical trials particularly where clinical outcomes or events cannot practically or ethically be measured. By using biomarkers to assess patient response, ineffective drug candidates may be terminated earlier in the development process in favor of more promising drug candidates.
Common examples of biomarkers include low-density lipoprotein (LDL) cholesterol for assessing risk for atherosclerosis and human estrogen receptor-2 (HER2) to assess breast cancer risk.
Overexpression of HER2 by breast cancer cells results in breast cancer that grows and spreads faster. Patients with tumors overexpressing HER2 are commonly treated with Trastuzumab, a therapeutic antibody that binds to HER2. Since Trastuzumab is only effective against HER2-positive tumors, HER2 serves as an important biomarker for the direction of treatment in breast cancer.
Learn more about our HER2 iLite reporter-gene assay.
As an important tool for informed decision, biomarker research can be influential across all phases of drug development, from drug discovery and preclinical evaluations to clinical development and post-marketing studies.
Furthermore, biomarkers are being used to optimize clinical trial designs and outcomes by identifying patient populations that are more likely to respond to a drug therapy or to avoid specific adverse events.
Svar offers a complete biomarker service tailored to your drug development project. In addition to practical, technical, and scientific support.
Wieslab Bioanalytical Services can help you from start to finish in finding the optimal solution that fits your project needs and budget. We know that each project has unique requirements that need to be catered to when forming a project strategy.
Our expert service scientists in combination with our portfolio of over 250 immunology markers put us in a unique position to perform sample analysis of biomarker protein concentration levels in nonclinical and clinical settings that meet your expectations concerning reporting times, sample handling, and storage conditions. The platforms we apply in biomarker studies are typically MSD, ELISA, and SIMOA.