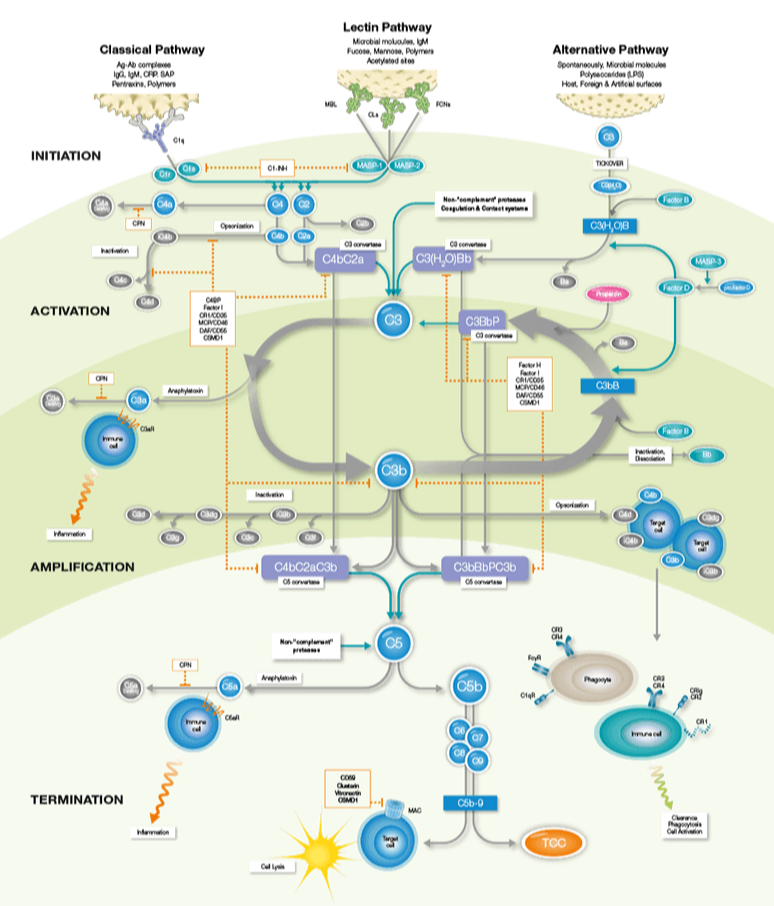
The role of Complement in drug development
It is becoming increasingly clear that an understanding of the complement system in relation to disease mechanism, and matching of drug modality and mode of action to the right disease and patient population is critical for drug development success.
Many pharma companies are working on drugs for manipulating complement activity via specific activation pathways, or isolated complement components but it is also important to keep in mind that different drugs can cause adverse effects based on how they unintendedly interact with single components or segments of the complement pathway, and how this in turn affects the rest of the complement system. Adverse effects related to the complement system can occur in response to various biomaterials or therapeutic cells.
The complement system can also be activated by artificial surfaces, for example during hemodialysis or cardiopulmonary bypass, resulting in increased levels of TCC. TCC is therefore well suited for studies of complement activation by biomaterials in medical devices and part of the recommended tests for assessing complement activation according to ISO standard 10993-4 for hemocompatibility testing.